
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Aa.32.
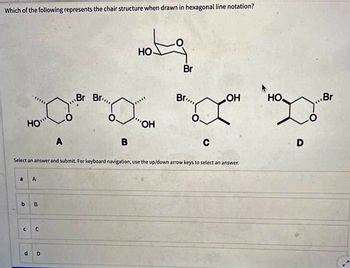
Transcribed Image Text:Which of the following represents the chair structure when drawn in hexagonal line notation?
HO
a A
b B
C C
A
d D
Br Br...
B
Hotg
HO
OH
Br
Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer.
Br...
C
LOH
HO
D
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the hybridization of the N atom in this molecule? What are the hybridizations of the other 4 atoms indicated? ............................ H-G-C-ċ-ċ-ö-H H H :N-H 2 E→:ö: HHO: A Harrow_forwardCan you please check if the skeletal and Lewis structure are correct as well as the hybridization. Can you also explain the bonding interactions between each pair of adjacent C atoms?arrow_forwardThe Lewis structure of an organic compound is shown below. Determine the orbitals used in bonding, as well as the number of sigma and pi bonds. 1サー= 10 H. N: H. 11 Total sigma bonds: [Select] Total pi bonds: [Select ] Write "none" if no orbital of that type is used to bond. Unhybridized orbital used to Hybridized orbital used to Atom bond bond [ Select ] [ Select ] [ Select] [ Select ] C # 2 [ Select ] [ Select ] [ Select ] [ Select]arrow_forward
- A Moving to another question will save this response. Question 23 What is the hybridization of the left-most carbon in the structure shown below? H₂C-N=C=Ö: O sp² spd sp sp O sp4 O 3arrow_forward16. State and explain the F-S-F bond angle in SF2. Your answer:arrow_forwardPls help ASAP. Pls do all asked questions.arrow_forward
- The instructions are to draw the molecule oriented as : Br going towards me while OH is going away from me. The compound is : CH3CH(Br)CH(OH)CH3 want to make sure I drew it correctlyarrow_forwardThe Lewis structure of an organic compound is shown below. Determine the orbitals used in bonding, as well as the number of sigma and pi bonds. 1ペー= 10 H 5. :N. 11 Total sigma bonds: [Select ] Total pi bonds: [Select] Write "none" if no orbital of that type is used to bond. Unhybridizedorbital used to Hybridized orbital used to Atom bond bond H. [ Select ] [Select] [ Select ] [ Select] N C #2 [ Select] [ Select] [ Select ] [ Select ]arrow_forwardIn a sample of bonds in the position. (Note: Remember that iodine () is larger and more likely to contribute to steric strain in the molecule.) iodocyclohexane (CH), one would expect to find most of the C-I O equatorial O trigonal pyramidal O tetrahedral Oplanar O axial Question 7 The molecule aniline is pictured here. Which of the following words could be used to describe an essential feature of aniline to someone who is familiar with chemistry, but not with this molecule? O aromatic O heterocycle O non-cyclic fused-ring system O alkane NH₂arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning