
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
33
Transcribed Image Text:Help
Save & Exi
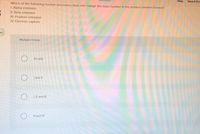
Which of the following nuclear processes does not change the mass number in the product element formed?
1. Alpha emission
II. Beta emission
III. Positron emission
IV. Electron capture
43
Multiple Choice
IV only
I and II
I, II, and II
IIl and IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Provide the major expected products: MgBr 1. CO2(s) 2. HCI, H2Oarrow_forward1. How many grams of Cu+ are present in 2.20 grams of copper(I) chromate? grams Cu+.2. How many grams of copper(I) chromate contain 3.24 grams of Cu+? grams copper(I) chromate.arrow_forward2Fe2O3 + 3C → 3CO2 + 4Fe D. How many grams of CO2 are produced from 11.42g Fe2O3?arrow_forward
- Balance and complete: HNO3(aq)+K2CO3(aq) Show your work!arrow_forwardIs the a( electrophillic or neucleophillic ) ( addition, elimination or substitute) reaction?arrow_forwardIdentify the type of each of the following reactions. 1. N2(g)+2O2(g)→2NO2(g) -- 2. CaCl2(aq)+2AgNO3(aq)→2AgCl(s)+Ca(NO3)2(aq) -- 3 .CS2(l)+3O2(g)→2SO2(g)+CO2(g) -- 4. H3PO4(aq)+3KOH(aq)→3H2O(l)+K3PO4(aq)-- options for each : --precipitation, acid-base, oxidation-reduction, or no reactionarrow_forward
- What is the product ?arrow_forwardC3tt&t 50273CO2+4H2 Howmonymolzs of Woter will be produced by the complete combusion of O,5 moles of propane, C3H8: nbusianarrow_forwardUsing the table provided, what is the enthalpy for the reaction shown below: 6H₂(g) + 4NO(g) + CH₂(g) → CO₂(g) + 2H₂O(l) + 4NH3 (9)arrow_forward
- Indicate the molecularity of the reaction represented in the figure diagram. Explain it. A A A Aarrow_forwardYou have a 11.5 mg sample of blood that contains various proteins. Hemoglobin is the only protein in the sample containing Fe. Hemoglobin contains 3.83% by mass of a compound called heme (C₃₄H₃₂FeN₄O₄, MW = 616.49 g/mol). You find that your 11.5 mg blood sample contains 34.9 micrograms of Fe. What is the mass percent of hemoglobin in your protein sample? -don't prematureley roundarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY