
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please show an explanation.
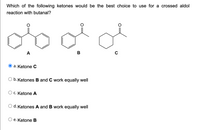
Transcribed Image Text:Which of the following ketones would be the best choice to use for a crossed aldol
reaction with butanal?
A
B
O a. Ketone C
O b. Ketones B and C work equally well
C. Ketone A
d. Ketones A and B work equally well
е. Ketone B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- e) emulsifiers are pretty important compounds for daily life, externally and internally to us humans. Describes the two parts of an emulsifier molecule, and how most emulsifiers work and what they actually do.arrow_forwardWhat does the term “free flowing” mean in organic chemistry lab?arrow_forwardOrganic Chemistry 1 Here is the professor's solution to the problem. The assignment has been turned in and the answer key has been shared with the class. So, this is the official answer key. However, I do NOT understand the solution. Please explain the answer in a clear step-by-step fashion to me. Explain the answer provided in the image in a clear fashion and as if I did not know much about organic chemistry. Question: Identify the isomeric relationship between the molecules of each pair depicted below. Your choices are diastereomers, enantiomers, identical, or constitutional. Explain your answer without using Fischer projections.arrow_forward
- 2. Two structural formulas for C₂H402 were provided in the Introduction for this lab. Draw a third structural formula of C₂H402.arrow_forwardDetermine the structures of the missing organic molecules in the following reaction: Y II OH X + H₂O H+ H* OH Note: Molecules that share the same letter have the exact same structure. In the drawing area below, draw the skeletal ("line") structures of the missing organic molecules X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Click and drag to start drawing a structure. C X :0 Ś m c+arrow_forwardThe name carbohydrate comes from the fact that many simple sugars have chemical formulae that look like water has simply been added to carbon. (The suffix hydrate from the Greek word hydor ("water") means "compound formed by the addition of water.") The actual chemical structure of carbohydrates doesn't look anything like water molecules bonded to carbon atoms (see sketch at right). But it is nevertheless possible to chemically extract all the hydrogen and oxygen from many simple carbohydrates as water, leaving only carbon behind. If you search the Internet for "reaction of sulfuric acid and sugar" you will find some impressive videos of this. Suppose you had 300.g of ordinary table sugar, which chemists call sucrose, and which has the chemical formula C12H22O11 . Calculate the maximum mass of water you could theoretically extract. Be sure your answer has a unit symbol, and round it to the correct number of significant digits.arrow_forward
- Please don't provide hand writtin solution...arrow_forward"Waterproof" nylon garments have a coating to prevent water from penetrating the hydrophilic fibers. The structure of a common nylon is shown below. H. H. Nylon-6,6 1st attempt See Periodic Table O See Hint Which structural features/groups in nylon-6,6 make it hydrophilic? Groups (5 items) (Drag and drop into the appropriate area below) Cof CH2 Nof NH Hof NH Hof CH2 O of C=O Categories Hydrophilic Hydrophobic Drag and drop here Drag and drop herearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY