
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
|
Optically active one compound. |
|
|
|
Optically active pair of diastereomers. |
|
|
Optically inactive one compound. |
|
|
Optically inactive pair of diastereomers. |
|
|
Optically active pair of enantiomers. |
|
|
Optically inactive pair of enantiomers. |
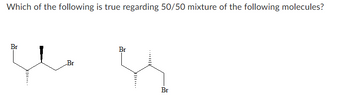
Transcribed Image Text:Which of the following is true regarding 50/50 mixture of the following molecules?
Br
R
Br
Br
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which statement is true about these Fischer structures? CH3 CH3 CH3 HO CH3 it: -H HO- -H H- -OH H- HO- -H- H- -OH H- HO- -- CH3 CH3 CH3 CH3 I II III IV O A I and II are identical (same stereoisomers). II and IV are identical. I and II are diastereoisomers. O D.II is a meso structure.arrow_forwardIdentify the relationship between these two structures. F OH НО. CH3 H CH3 H H₂C- H₂CH Diastereomers The same compounds O Unrelated compounds O Enantiomers יד -H F Iarrow_forwardWhich of the following is the definition of a pair of enantiomers? a. A pair of structures that are superposable mirror images of one another b. A pair of stereoisomers that are non-superposable mirror images of one another c. A pair of stereoisomers that are not mirror images of one another d. A pair of stereoisomers that have equal specific rotations Group of answer choices a b c darrow_forward
- C. Smelling Chirality. Carvone Olfactory receptors in our noses, used for the detection of odors, are chiral. Therefore, in some cases, we can tell the difference between enantiomers by their odor. Carvone is one such compound that we can distinguish between the R and S enantiomer by their odor. 1. Identify the chiral center in carvone and draw the R and S enantiomers in the indicated boxes. 2. Smell both of the enantiomers of carvone and provide a brief description of their odors in the same boxes. Carvone is perfectly safe to smell as both enantiomers are found in various foods. R-carvone S-carvonearrow_forwardConvert the ball-and-stick model to a Fischer projectionarrow_forwardWhat is the relationship between the following two molecules? a) Same molecule Ob) Enantiomers Oc) Diastereomers Structure A d) None of the above Structure Barrow_forward
- There are nine constitutional isomers of molecular formula C7H16. 1) What is the unsaturation number of each of these compounds? 2) Draw five constitutional isomers, where two of these are chiral compounds. 3) For the three achiral constitutional isomers, provide the correct IUPAC names. 4) For the two chiral constitutional isomers, draw in Fischer projection formula the enantiomers of each. 5) Name, according to IUPAC standards, each of the enantiomers you drew in #4 above.arrow_forwardHow many sp3 stereocenters and diastereomers(2n) does Miglitol have?arrow_forwardDraw the mirror image in Fischer Projection ("flat representation").arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY