
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
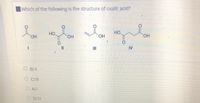
Transcribed Image Text:Which of the following is the structure of oxalic acid?
HO.
HO.
HO.
HO.
HO,
HO.
%3D
IV
B) ||
C) II
O A)I
O D) IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hand written solutions are strictly prohibitedarrow_forwardComplete the following table. Be sure to show calculations in detail.arrow_forward) 43.IML of o-68m strontium hydro xide is required to Q3 Solve the indicated problem 43.1ML 0f o.68M hydroxide is required to strontiam de composi hón of the accd? ollo 114.6mL of O.23 M calcium hudro xide is required to neatralise 65.omL Of acetic a cid. what is the concen tration Of the acid? 52.3m2 of 8.26M hydrobromic acid is required to neatralize 75.0mL of a 3olution hydro xide. what is the of barium hydroxide. what is fhe Of the acid? っdap Concentra hön contout tONt sbiroub Nodhp toarrow_forward
- 19arrow_forward21. Identify the Lewis acid in the reaction. wwo O A) SO3 O B) o?- Og so4?- O D) None of these. O EJ All of these.arrow_forward20.) A 0.64 M solution of the weak base hydrazine (N2H4) is found to be 0.16% ionized at equilibrium.What is the value of Kb for hydrazine?a. 1.0x10-3 b. 5.77 c. 1.6x10-6 d. 6.7x10-4 e. 1.0x10-1arrow_forward
- Lost the risk and the benefit of the following reactionarrow_forwardWhich of the following is Lewis acid? H. H. H-B-H NH, H- - II II both I and Ilarrow_forwardTo what category of basicity does CO32- belong? Select one: a. weakly basic b. moderately basic c. feebly basic d. nonbasic e. strongly or very strongly basicarrow_forward
- Which of the following salts will result in basic solution in water? A. NaCl B. Na2S C. BaCl2 D. FeCl3 E. CuSO4arrow_forward69. What is [OH-] in aqueous solutions with the following [H3O+] concentrations? a. 5.5 * 10^-2 b. 9.4 * 10^-5 c. 2.3 * 10^-7 d, 6.6 * 10^-12arrow_forwardA solution with a pOH of 11.70 has an [H3O+] ofarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY