
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
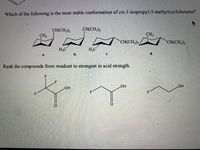
Transcribed Image Text:Which of the following is the most stable conformation of cis-1-isopropyl-3-methylcyclohexane?
CH(CH3)2
CH(CH;)2
CH3
CH3
CH(CH3)2
CH(CH,)2
H3C
H3C
d.
Rank the compounds from weakest to strongest in acid strength.
.F
OH
F
Transcribed Image Text:Indole can function as a Bronsted-Lowry acid in the presence of strong bases. Complete the reaction,
using hydroxide as the base showing electron flow with arrows that demonstrates this reactivity of indole.
НО
N.
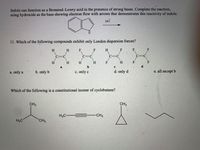
11. Which of the following compounds exhibit only London dispersion forces?
H.
H.
F
F
H
F
F
F
C=C
C=C
C=C
C=C
H
H.
H.
H
H.
F
F
b
d.
a. only a
b. only b
c. only c
d. only d
e. all except b
Which of the following is a constitutional isomer of cyclobutane?
CH3
CH3
H3C
-CH3
H3C
CH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which is a pair of enantiomers? Explain why.arrow_forwardCH₂CH₂CCH₂CH₂CH, H₂O Br₂ tert-BUO CH₂CH₂CH₂CH₂Br CHỊCH C—CH CHỊCH; CH₂CH₂CH₂CH.Br CH₂CH3 tert-BuO CH₂CH₂CH=CH₂ H₂O NaNH, CH3CH₂Br -CH₂CH₂CHCH₂ CH;CH C=CCHỊCH, Br excess H₂SO4 Re H₂SO4 CH;CH,C=CH CHỊCH C=Carrow_forwardPlease answer parts G,H,Iarrow_forward
- Nonearrow_forwardThe structure of 4 isomers of ketopentose are shown. 1) select every structure that is a diastereomer of structure D A, B, or C? 2) select every structure that is a enantiomer of structure C A, B, or D 3) select every structure that is a stereoisomer of structure C A, B, or Darrow_forwardChoices a.3 b. 2 c. 4 d. 1arrow_forward
- Which of the following structures represents the most stable conformation of cis-1-ethyl-2-methylcyclohexane? Select one: Me O a. Et O b. c. Me. Et d. Me Et Which of the following structures would be the most stable carbocation? Select one: O a. O b. Ос. O d.arrow_forwardWhich one of the following is the s-trans conformation of (E)-2-methyl-2,4-hexadiene? A) Select one: OA. B OB. D OC. C OD. A n B) C)arrow_forward2. a) Draw the most stable chair conformation of cis 1-isobutyl-2-methylcyclohexane. b) Draw a Newman projection of the best conformation of 2,3-difluorobutane looking down the C2-C3 bond. 3. Compound X has the formula C46H90O4. Compound X reacts with excess H2/Pd to give C46H9204. How many rings does compound X have? Show how you got your answer. 4. For each pair state whether the two are a) the same molecule, b) different compounds that are not isomers, c) constitutional isomers, d) diastereomers, or e) enantiomers. A) B) ÇH3 CH3 Br Br HS CI CI- SH H Br Br CH3 CH3arrow_forward
- 1) The most stable chair confirmation ofarrow_forwardGive the relationship between each pair of molecules below (constitutional isomers, diastereomers, enantiomers, or identical). Note that molecules may not necessarily be shown in the same conformation. 3. OH ОН ОН ОН (a) (b) ОН H OH (c) CH3 .CI (d) Н. .CH3 CH3 CH3 ОН TCI H H CI OH F Br Br ОН ОН ОН ОН (e) (f) CI ОН ОН (g) .cO NH2 NH2 .. O II S:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY