
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
q43
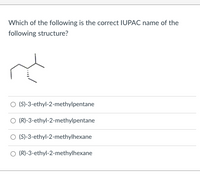
Transcribed Image Text:Which of the following is the correct IUPAC name of the
following structure?
O (S)-3-ethyl-2-methylpentane
O (R)-3-ethyl-2-methylpentane
O (S)-3-ethyl-2-methylhexane
O (R)-3-ethyl-2-methylhexane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- which CH₂ +HI →→→ ?? Whal law used Draw majors minerarrow_forward9) I have circled six different species in the attachment table of standard reduction potentials. Just consider the half-reaction that involve the circled species. Which of the circled species are oxidizing agents? Which is the strongest oxidizing agent? Which is the weakest oxidizing agent? Which of the circled species are reducing agents? Which is the strongest reducing agent? Which is the weakest reducing agent?arrow_forwardLICH Aadic envirment K Oarrow_forward
- eleriged enxim loe lenipho er) olebydi0 obholrO muhss to eesM DogecapAqua ne (s mod yewene) e bede) bede) m phoaphate hate dodeclydrate heacted olorigaon9 mulbo2 to Ineoie eutxim isa lenipho adt.ni oimbydeobo0 ni eterbyria ebhoirO rue oubxim ilse lsnipno en 3. Octane is one of the hydrocarbon components in gasoline, which is the main ) fuel source for automobiles. Consider the combustion reaction of octane (C8H18) and determine how many grams of oxygen (O2) and carbon dioxide (CO2) are produced when 800 pounds of octane are reacted. ni elsibyriid ebholrO mune8 lo inegte Aquete tu eferigaori9 muibo to ineone nte uxim ilsa lanigho ert enuhxim ilea lanigno erli ni olenbvrisoebo0 belceur o 84 | Page 13arrow_forwardComplete each row of the table below by filling in the missing prefix. 1 | Hz 3 10 Hz %3D 1 Hz 10 Hz -6 1 Hz 10 Hz %3D -1 1 Hz 10 Hzarrow_forwardC20H2³CIN²04 +2 HCIO4.arrow_forward
- 30 70- 60- 50- 40- 30 20 10 0₁ 4000 Ророжа Structue nd, C₂H₂1N 3500 иде Sp 3000 2500 18 +2-20 го- 2000 1800 hu 1600 Ф тро 1400 1200 1000 800 6 Н 6 Н 9 Н 600 Уarrow_forwardplease answer question 4 and 5arrow_forwardtab caps lock You have a 11.5 mg sample of blood that contains various proteins. Hemoglobin is the only protein in the sample containing Fe. Hemoglobin contains 3.83% by mass of a compound called heme (C34H32FeN4O4, MW = 616.49 g/mol). You find that your 11.5 mg blood sample contains 29.7 micrograms of Fe. What is the mass percent of hemoglobin in your protein sample? Tap here or pull up for additional resources Q A NO 2 W S #3 E D GA 4 R % 5 F Question 9 of 17 T 6 G Y & 7 B H 8 N 9 4 +/ V Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY