
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
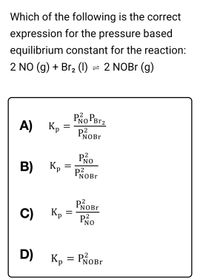
Transcribed Image Text:Which of the following is the correct
expression for the pressure based
equilibrium constant for the reaction:
2 NO (g) + Br, (1) = 2 NOBr (g)
А) Кр
Po Perz
PROB.
NOBr
Po
B)
Kp
P2
NOB.
PROB.
Kp
C)
PNO
D)
Кр
NOBr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The equilibrium system 2A <-> 2B + C has a very small equilibrium constant: K = 2.6 ´ 10–6. Initially 3.0 moles of A are placed in a 1.5-L flask. Determine the concentration of C at equilibrium.arrow_forwardAt a certain temperature, the reaction PCl5(g) <====> PCl3(g) + Cl2(g) has an equilibrium constant Kc=5.8x10^-2 a) calculate Kc for the synthesis of 2 moles of PCl5(g) at the same temperaturearrow_forwardConsider the equilibrium system described by the chemical reaction below, which has a value of Kc equal to 7.1 × 10-4 at a certain temperature. If a solid sample of CaCro4 dissolves in solution, what will the equilibrium concentration of Ca2* in the solution be? СacrOa(s) 3D са?" (аq) + CrO,? (aq) 2- 1 2 3 NEXT > Based on the given values, set up ICE table in order to determine the unknown. CaCrO4(s) Ca²*(aq) CrO,2 (aq) + Initial (M) Change (M) Equilibrium (M)arrow_forward
- Im a student plz helparrow_forwardConsider the reaction. А(аq) — 3 В(аq) K. = 2.15 x 10-6 at 500 K If a 4.80 M sample of A is heated to 500 K, what is the concentration of B at equilibrium? [B] =arrow_forwardhydrogen sulfide is a pungent, poisonous gas. At 1400 K, an equilibrium mixture was found to contain 0.013 mol/L of hydrogen, 0.18 mol/L of hydrogen sulfide and an undetermined amount of sulfur in the form of S2 gas. If the value of Kc is 2.40 x10^-4 at this temperature what concentration of S2 is present at equilibrium?arrow_forward
- For the reaction in a sealed container at a constant temperature,2NO(g)+Br2(g)<--->2NOBr(g)the initial concentrations are 4.50 M NO, 4.60 M Br2, and no NOBr. At equilibrium, [NOBr] = 2.20 M. Calculate the value of K under the reaction conditions.arrow_forward+O2 (8) 2 SO, (8) A,2.000 Lflask was initially filled with,0.0400 mol SO and 0.0200 mol O, and was allowed to reach equilibrium. If the flask contained, 0.0296 mol SO, at equilibrium: Consider the following: 2 SO, (e) 2 (g) 32 3. al Calculate the moles of each substance present at equilibrium. b| Calculate the equilibrium constant, K. + O2 (3) = 2SO, (8) 3 (g) c Calculate the equilibrium constant, K, for the following: 2 SO, +2O2(8) = 4 SO, (3) 2 (g) d| Calculate the equilibrium constant, K, for the following: 4 SO, (e) of HCN CHEMISTRY 1516Larrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY