
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
The diagram above represents the
Which of the following is closest to the
Thank you very much!
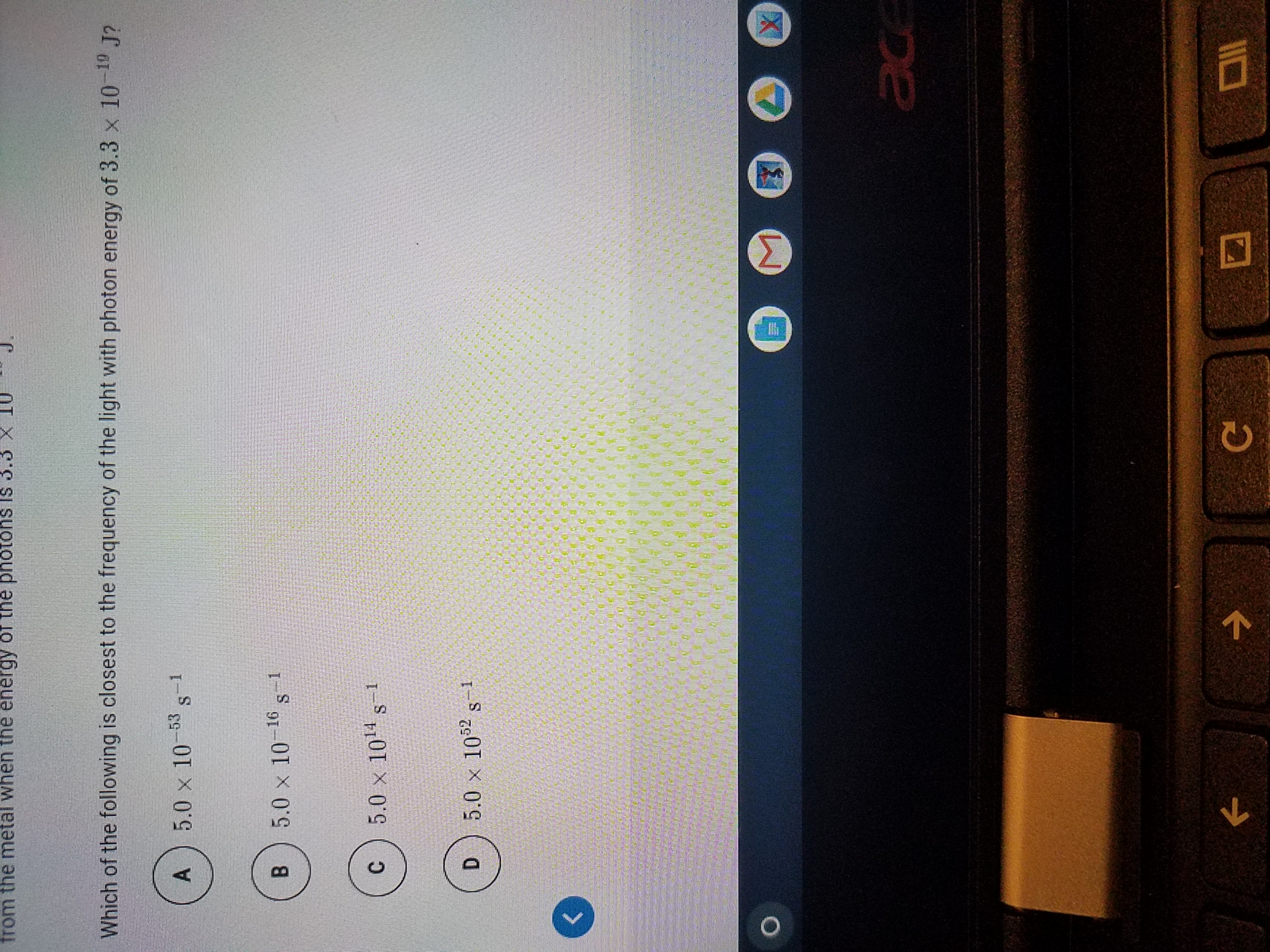
Transcribed Image Text:Which of the following is closest to the frequency of the light with photon energy of 3.3 x 10-19 J?
A
5.0 x 10-53 s-1
B.
5.0 x 10 1 s
-16
5.0 x 1014 s-1
5.0 x 1052 s-1
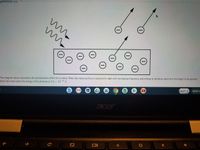
Transcribed Image Text:The diagram above represents the photoelectric effect for a metal. When the metal surface is exposed to light with increasing frequency and energy of photons, electrons first begin to be ejected
from the metal when the energy of the photons is 3.3 x 10 " J.
国
EXTD
acer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In scenario A, visible light has a wavelength of 690.3 nm. Determine its frequency, energy per photon, and color. frequency: s-1 energy per photon: The visible light in scenario A is Jarrow_forwardA Li2+ ion absorbs a photon with a frequency of 6.66 x 10^14 Hz. If the energy of the final state of Li2+ was -7.85 x 10^19 J, calculate the energy of the initial state.arrow_forwardCalculate the wavelength(nm) of light that strikes a metal with a threshold frequency of 2.84 x 1014s-1 and ejects an electron with a kinetic energy of 5.48 x 10-19J.arrow_forward
- The work function (φ) for a metal is the amount of energy needed to eject an electron from the surface of the metal via the photoelectric effect. For nickel φ = 8.25 x 10-19J. Will a photon with a wavelength (λ) of 250.0 nm have enough energy to eject an electron from nickel? If the speed of an electron in the n = 4 energy level of a hydrogen electron is 2.3 x 106 m/s, what is the de Broglie wavelength of this electron in nanometers (the mass of an electron is 9.10938 x 10-31 kg). Note: 1 ? = 1 ?? ∙?2 An F-15 Strike Eagle weighs in at roughly 32,000 kg fully loaded. If an F-15 Eagle is traveling at 3,017 kilometers per hour, what is the de Broglie wavelength associated with the aircraft at this speed in nanometers?arrow_forwardThe minimum frequency of light needed to eject electrons from a metal is called the threshold frequency, ?0. Find the minimum energy needed to eject electrons from a metal with a threshold frequency of 3.20×1014 s−1. With what maximum kinetic energy will electrons be ejected when this metal is exposed to light with a wavelength of 285 nm?arrow_forwardIf a laser emits 5.4 x 1019 photons per second and transfers a total of 87.06 J of energy over 1.93 seconds, what is the wavelength of the emitted light in nm?arrow_forward
- The electron in an (unbound) hydrogen atom (H(g)) is excited from the ground state to the n=3 state.Which of the following statements are true and which are false. 1. it takes less energy to ionize the electron from n=3 than it does from the ground state.2. The wavelength of the light emitted when the electron returns to the ground state from n=3 is the same as the wavelength of light absorbed to go from n=1 to n=3.3. the electron is farther from the nucleus on average in the n=3 state than in the ground state.4. The first excited state corresponds to n=3.5. The wavelength of light emitted when the electron drops from n=3 to n=2 is shorter than the wavelength of light emitted if the electron falls from n=3 to n=1.arrow_forwardA metal surface has a threshold energy of 225 kJ/mol. After being exposed to light with frequency 7.3 × 1014 Hz , with what velocity will electrons be ejected?arrow_forwardDetermine the energy of a photon, in J, with a wavelength of 294 nm. (h = 6.626 × 10⁻³⁴ J • s and c = 3.00 × 10⁸ m/s) What is the energy (in J) of a mole of photons that have a wavelength of 681 nm? (h = 6.626 × 10⁻³⁴ J • s and c = 3.00 × 10⁸ m/s)arrow_forward
- If a laser emits 5.0 x 1019 photons per second and transfers a total of 80.58 J of energy over 2.06 seconds, what is the wavelength of the emitted light in nm?arrow_forwardThe minimum energy required to remove an electron from a certain metal is 3.7 x 10– 19 J. If the metal surface is struck by light having a wavelength of 5.00 x 102 nm, what is the kinetic energy of the electron ejected from the metal?arrow_forwardAn electron in a hydrogen atom falls to the n=2 energy level and emits a photon with a wavelength of 410.0 nm. What was the electron's initial energy level?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY