
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Question is for practice and not graded. Please elaborate on answer. Thank you.
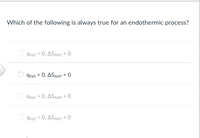
Transcribed Image Text:**Question:**
Which of the following is always true for an endothermic process?
**Options:**
- \( q_{\text{sys}} < 0, \Delta S_{\text{surr}} > 0 \)
- \( q_{\text{sys}} > 0, \Delta S_{\text{surr}} < 0 \)
- \( q_{\text{sys}} > 0, \Delta S_{\text{surr}} > 0 \)
- \( q_{\text{sys}} < 0, \Delta S_{\text{surr}} < 0 \)
**Explanation:**
- **\( q_{\text{sys}} \):** Represents the heat absorbed or released by the system.
- **\( \Delta S_{\text{surr}} \):** Represents the change in entropy of the surroundings.
**Correct Answer:** The correct option is the second one: \( q_{\text{sys}} > 0, \Delta S_{\text{surr}} < 0 \), indicating that during an endothermic process, the system absorbs heat (positive \( q_{\text{sys}} \)), leading to a decrease in the entropy of the surroundings (negative \( \Delta S_{\text{surr}} \)).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- I need help completing this I just need the formula and names of each box, there are some that I did.arrow_forwardI took a picture of what I put for the answer, however for some reason that is still wrong. Please let me know how I can fix it! Thank you!arrow_forwardH OH 1. NaBH₂, MeOH 2. Nalo Ketone AlkyLOHI I. Harrow_forward
- Based on the information given, identify the most significant error in the preparation of Graph 2. A) The title appears in a font which is too small. B) The best-fit line does not intersect any data point. C) There is wasted space on the graph. D) The x-axis scale divisions are uneven.arrow_forward6/3 Li ------> 4/2 He + _____________?arrow_forwardPart A incorrect you filled in 4 of 5 blanks incorrectlyarrow_forward
- help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardIn the following sub-questions, use your book and notes to generate an argument for each model of light. Remember, on the first day of class we discussed that argumentation requires: ● a claim (which I've given), • evidence (which you should look up in the form of data or scientific principles) and reasoning that connects the evidence to the claim (which you should generate from your understanding so far).arrow_forwardPart A transcript When glucose (C6H12O6 (s)) is consumed, it reacts with O₂ gas in the body to produce gaseous carbon dioxide and liquid water. Enter the balanced chemical equation for the reaction. Express your answer as a chemical equation including phases. 0 ΑΣΦ ? A chemical reaction does not occur for this question. Submit Previous Answers Request Answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY