
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
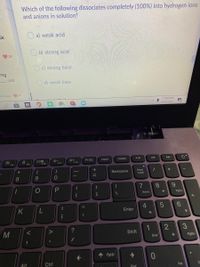
Transcribed Image Text:Which of the following dissociates completely (100%) into hydrogen ions
and anions in solution?
ak
a) weak acid
O b) strong acid
27
O c) strong base
ong
salt
d) weak base
19 votes
30
11:44 AM
4/23/2021
PrtSc
Insert
Delete
F8
F9
F10
F11
F12
+
Backspace
Num
Lock
%3D
8
9.
7
8
Home
PgUp
%3D
K
Enter
M
1
Shift
3
End
PgDn
PgUp
Ctrl
Ins
End
->
4-
+ ||
1O
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. The pH of solution A1, solution A2, solution A3 and solution A4 are 1.0, 2.0, 3.0, and 4.0, respectively, which one is the strongest acid? The molarities of the 4 acid solutions are the same. O O (A) A2 (B) A4 (C) A1 (D) A3arrow_forwardQuestion 8 of 20 Sub Complete the balanced molecular reaction for the following weak base with a strong acid: NaCIO2(aq) + H2SO«(aq) – 3- 2. 2+ 3+ 4+ 04- 1 6 7 8. 9. 01 D 07 9. 2 3 4. 6. (s) (1) (g) (aq) -> Na H2O H3O* OHT H. CI • x H2O Delete Reset W ? Questions prep ok étv A 山 T。 OCT 4. MacBook Pro 8. 11arrow_forwardQuestion 21 Which of the following statements is true for a basic solution: O [H*) > 107 M O none of the above O pH < 7 O JOH'] < 107Marrow_forward
- How do you write BrØnsted-Lowry equations for acid-base equilibrium reactions for both monoprotic and polyprotic acids and bases? could you use a diploma question as the answer ( grade 12 )arrow_forwardQUESTION 13 What is the pH strength of the following structure? SH OA Weak acid OB. Strong acid OC. Neutral OD.Weak base O E Strong basearrow_forwardQUESTION 18 Consider three generic acids with the relative strengths: HX>HY>HZ Which of the following is the weakest conjugate base? O x- OY O Z O Not enough informationarrow_forward
- Consider three generic acids: HX, HY, and HZ. -1 HX OH OX Arrange the acids according to strength. WWW HZ WWW HZ OH OZ WE HY E CHY CHOYarrow_forward8:19 AM Wed Mar 1 Question 25 of 26 What is the percent ionization of H₂NNH₂ in a solution with a concentration of a 0.590 M? (Kb = 1.3 × 10-) itional resourcesarrow_forwardChemistryarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY