
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
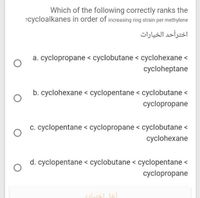
Transcribed Image Text:Which of the following correctly ranks the
?cycloalkanes in order of increasing ring strain per methylene
اختر أحد الخيارات
a. cyclopropane < cyclobutane < cyclohexane <
cycloheptane
b. cyclohexane < cyclopentane < cyclobutane <
cyclopropane
c. cyclopentane < cyclopropane < cyclobutane <
cyclohexane
d. cyclopentane < cyclobutane < cyclopentane <
cyclopropane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 8. The heat of combustion (per CH₂) of several cycloalkanes is listed below. Based on the data given, which of these cycloalkanes would be considered most stable? Heat of combustion (kJ/CH₂) -686.5 -664.0 -663.6 -659.0 (A) cyclobutane (C) cyclooctane Cycloalkane cyclobutane cyclopentane cyclooctane cyclopentadecane (B) cyclopentane (D) cyclopentadecanearrow_forwardThe following names are all incorrect or incomplete, but they represent real structures. Draw each structure and name it correctly.arrow_forwardName the alkenes below.arrow_forward
- 28. Which two of the following four molecules are structural isomers? * CH3 H3C- CH2 H3C CH CH2 CH3 CH CH3 but-2-ene methylcyclopropane butane ethylcyclopropane Omethylcyclopropane and butane but-2-ene and ethylcyclopropane Omethylcyclopropane and but-2-ene Obutane and ethylcyclopropanearrow_forwardwhat are the names of these alkanes?arrow_forwardPlease provide the reason why the chosen answer is correct!arrow_forward
- Macmillan Learning H&C. CH2 H&C CH HC Consider alkene 2. CH3 ΤΟ =CH2 H2 H₂C 생 Consider alkene 3. H₂C H.C CH3 CH2 =CH2 alkene 1 name: 3-4-dimethyl-1-hexene Incorrect alkene 2 name: 3-methylhept-1,3-diene Incorrect alkene 3 name: CH2 2-ethylbut-1,3-diene Incorrect Attempt 3arrow_forwardWhich of the following correctly ranks the cycloalkanes in order of increasing ring strain per methylene? cyclopropane < cyclopentane < cyclobutane < cyclohexane cyclohexane < cyclopentane < cyclobutane < cyclopropane cyclopentane < cyclopropane cyclobutane cyclohexane www cyclopropane < cyclobutane < cyclohexane < cycloheptane FET HEAL TEILELF OF TEMELETE cyclopentane < cyclobutanearrow_forwardWrite the IUPAC name of the following ALKYNES: CHΞCH CH3-CH2-CΞCH CHΞC-CH|CH3|-CH2-CH3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY