
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
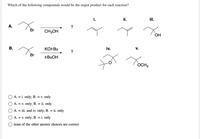
Transcribed Image Text:Which of the following compounds would be the major product for each reaction?
i.
ii.
ii.
А.
?
Br
CH;OH
OH
KO-Bu
iv.
V.
?
Br
t-BUOH
OCH3
A. = i. only; B. = v. only
A. = v. only; B.
ii. only
A. = iii. and iv. only; B. = ii. only
A. = v. only; B. = i. only
none of the other answer choices are correct
B.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What product(s) result in the following reaction? Select all that apply. CHCl3. KotBu А. D. G. H H H H CI CI H B. E. H H. H CI CI H C. F. H Harrow_forwardWhat is the major organic product of the following reaction sequence? Ol Oll O III OIV NH₂ II CN Br: 1. NaCN 2. LIAIH4 3. H₂O* III NH₂ IV. :0: NH₂arrow_forwardWhich reagent(s) are best used to carry out the following reaction? ? a. H2CrO7 b. 1. propanol, NaOH; 2. HCI c. 1. CH3CH2MgBr, THF; 2. HCI d. H2SO4, H2O a b darrow_forward
- 3.a. Give the mechanism(s) and product(s) for the reaction below. Show stereochemistry. D HI.. CO HI Me 3.b. Label the major and minor products in 3.a. H₂SO4, H₂Oarrow_forward6. Provide the structure for the major product in the following reactions. b. P f. OH g. Lia * Oia ملی CI 1. SOCI₂, pyr. 2. to Br 2 1. LIAIH4 (xs) 2. H₂O 1. LIAI(OR) 3H 2. H₂O 2. NH₂ Et₂CuLi 1. EtMgBr (xs) 2. H₂O 1. Mg 2. CO₂ 3. H* 4. SOCI₂, pyr 1. [H], NaBH3CN, EtNH₂ CI pyridinearrow_forwardI need help with thisarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY