
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
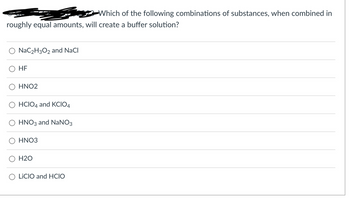
Transcribed Image Text:Which of the following combinations of substances, when combined in
roughly equal amounts, will create a buffer solution?
NaC₂H3O2 and NaCl
HF
HNO2
HCIO4 and KCIO4
HNO3 and NaNO3
HNO3
H2O
LICIO and HCIO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You are asked to find the pH of each solution described below. A 0.50 M solution of HBrO3 A 0.030 M solution of Ca(OH)2 A 0.50 M solution of NH4Br A solution that is 0.20 M in NH3 and 0.35 M in NH4NO3 after the addition of a small amount of NaOH A solution that is 0.40 M in HCOOH and 0.30 M in NaHCOO A 0.25 M solution of CH3NH2 Options: A.weak acid B.strong acid C.weak base D.strong base E.acid salt F.basic salt G.buff by it self H.buff after add strong base I.buff after add strong acidarrow_forwardClassify the following salts as acidic/basic/pH neutral and write a hydrolysis equation for them where appropriate. salt Acidic/basic/neutral Hydrolysis equation if applicable NaNO3 CsF NH4Cl KCl Ba(CH3COO)2 CH3NH3Iarrow_forwardWhat component do you need to add to H2CO3 to make a buffer? H2O OH- H3O+ HCO3-arrow_forward
- Consider that 20.0 mL of 0.10 M HA (an arbitrary weak acid, Ka= 1.5 × 10−6) is titrated with 0.10 M NaOH solution. The ionization of HA in water occurs as the following.HA (aq) + H2O(l) ⇌ A-(aq) + H3O+ (aq)The neutralization reaction between HA and NaOH can be expressed as the following. HA (aq) + NaOH (aq) ® NaA (aq) + H2O (l) Answer the following questions. A) What will be the initial pH of the 0.10 M HA solution?arrow_forwardCalculate the pH of each of the following buffered solutions: a) 0.25 M acetic acid CH3COOH (Ka = 1.8 x 10-5) and 0.10 M sodium acetate CH3COONa b) 0.50 M C₂H5NH2 (Kb = 5.6 x 104) and 0.50 M C₂H5NH3C1arrow_forwardWhich mixture of equal volumes of the following solutions will produce a buffer solution? A. 0.5 M CH3COOH and 0.5 M NH4Cl B. 1.0 M NH3 and 0.5 M HNO3 C. 0.5 M NH4Cl and 0.5 M NaOH D. 0.5 M CH3COOH and 1.0 M NaOH E. 0.5 M CH3COOH and 0.5 M NaOHarrow_forward
- Which of the following aqueous mixtures would be a buffer solution? Select one: CH3COOH, NaH₂PO4 O HSO4, HSO3 H₂PO4, HPO4²- O H₂CO3, HSO3™arrow_forwardWhich of the following pairs of substances can be used to make a buffer solution? a. HBr & KOH b. CH3COOH & HF c. HClO3 & KC2H5COO d. HCOOH & HBr e. NaHCOO & LiC6H5COOarrow_forwardWhich one of the following combinations cannot function as a buffer solution? NaHSO4 and H2SO4 KHCO3 and H2CO3 NaHS and H2S Na2HPO4 and NaH2PO4 NH3 and NH4Iarrow_forward
- Which of the following combinations could not be used to make an effective buffer? O HNO2 and NaNO2 O NH,Cl and NH3 O CH,CO,H and NaCH,CO2 O NaHSO3 and NazSO4arrow_forwardWhich of the following mixtures will be buffered when dissolved in a liter of water? 1 mol Calcium hydroxide and 3 mols hydroiodic acid 1 mol of sodium chloride and 2 mols of hydrochloric acid 4 mols of ammonia and 4 mols of hydrochloric acid 2 mols of hydrobromic acid and 1 mol of sodium hydroxide 2 mols of phosphoric acid and 2 mols of sodium hydroxidearrow_forwardDescribe how you would prepare a 250.0 mL buffer solution that has a pH of 7.40 and has a H2PO4 − concentration of 1.0 M. You only have the following materials available. Materials/Chemicals: 1 L jug of deionized water 250.0 mL volumetric flask 500 mL bottle of 1.0 M H3PO4 500 g bottle of NaH2PO4 500 g bottle of Na2HPO4 500 g bottle of Na3PO4 500 g bottle of HFarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY