
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
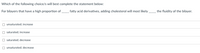
Transcribed Image Text:Which of the following choice/s will best complete the statement below:
For bilayers that have a high proportion of fatty acid derivatives, adding cholesterol will most likely
the fluidity of the bilayer.
unsaturated; increase
saturated; increase
saturated; decrease
O unsaturated; decrease
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Explain how the following affect membrane fluidity:– Level of phospholipid tail saturation– Level of cholesterol– Phospholipid tail lengtharrow_forwardWhich of the following is not a lipid link that anchors membrane-associated proteins to the bilayer? Thioether-linked prenyl anchors. Ester-linked triacylglycerol anchors. Glycosyl phosphatidylinositol anchors. Thioester-linked fatty acyl anchors. Amide-linked myristoyl anchors.arrow_forwardPhospholipids are the only molecule type which make up lipid bilayers in plasma membranes True or Falsearrow_forward
- Which of the following will decrease diffusion rate (a.k.a. rate of flux) of a particle across the plasma membrane? O Applying a polymer that makes the particle more hydrophobic. O Changing the diameter of the particle from 1uM to 10uM. O Both of these will decrease diffusion rate ONeither of these will decrease diffusion ratearrow_forwardThe cytoplasm of a certain cell is composed of a solution that is 98% water, 2% solutes. Consider the solution shown in the beaker in this picture: FOOE OOS Foor solution. [Select] Cytoplasm is 98% water, 2% solutes. The solution in the beaker would be considered when compared to [Select] The plasma membrane is impermeable to solutes. Beaker solution is 98% water, 2% solutes the cell. We would expect that the cell would [Select] if it was placed in the would account for whatever changes might occur to the volume of the cytoplasm of the cell when it is placed in the solution.arrow_forwardWrite 2 (any) functions of membrane carbohydrates and explain the functions.arrow_forward
- Which statement is correct about the phospholipids in membranes? The polar heads face inward The non-polar tails face outward The non-polar tails are hydrophilic A bilayer forms with the heads facing outward and the tails facing inwardarrow_forwardThe figure below illustrates a portion of a cell membrane. X denotes a membrane-spanning protein channel. Illustration of a partial cell membrane Which of the following is most likely true about the structure of X X A The upper and lower ends of X are hydrophilic and its middle is hydrophobic. C D The upper and lower ends of X, as well as its middle, are all hydrophobic. The upper and lower ends of X are hydrophobic and its middle is hydrophilic. The upper and lower ends of X, as well as its middle, are all hydrophilic.arrow_forwardEukaryotic membrane fluidity can be increased by which of the following mechanisms? increasing the number of fatty acids attached to glycerol in membrane lipids decreasing the number of branched chain fatty acids of membrane lipids decreasing the number of carbons in the fatty acids of membrane lipids decreasing the number of membraned organelles in the eukaryotic cell increasing the degree of saturation in the fatty acids of membrane lipidsarrow_forward
- Fat is stored in adipose cells in the body, and glycoproteins are produced by liver cells. Which of the following is likely to be more prominent in adipose cells than in liver cells? A B с D cell membrane smooth endoplasmic reticulum nucleus rough endoplasmic reticulumarrow_forwardWhich of these can never cross the lipid bilayer without the help of a transporter or a channel? O CO2 O 02 O water O Na+ O all of the above can diffuse through the lipid bilayer without transporter proteins or channelsarrow_forwardIn an experiment, a 0.001 (mole fraction) solution of polysaccharide in water is made and is placed in the compartment A. Compartment B is filled with pure water. The two compartments are separated by a porous semi-permeable membrane that allows the exchange of water molecules between the two compartments, but not that of the larger polysaccharide molecules. part 1: Show that the chemical potential of water in compartment A is lower than that in compartment B by 2.48 J/mol.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON