
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
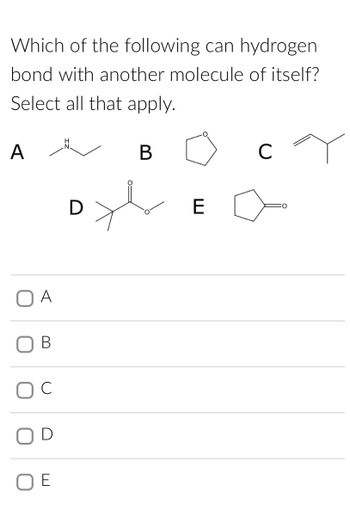
Transcribed Image Text:**Question:**
Which of the following can hydrogen bond with another molecule of itself? Select all that apply.
**Options:**
- **A**
- Structural Formula: Ethylamine (contains an -NH group)
- **B**
- Structural Formula: Tetrahydrofuran (contains an ether oxygen in a five-membered ring)
- **C**
- Structural Formula: Isobutylene (simple alkene)
- **D**
- Structural Formula: Isopropyl acetate (contains an ester functional group)
- **E**
- Structural Formula: Cyclopentanone (contains a ketone group in a five-membered ring)
**Answer Choices:**
- □ A
- □ B
- □ C
- □ D
- □ E
**Explanation:**
For hydrogen bonding, a molecule must typically contain hydrogen attached to a highly electronegative atom like nitrogen, oxygen, or fluorine.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of following species are capable of hydrogen bonding among themselves? Select all that apply or None of the above. NaCl C3H8 C4H10 CH3CONH2arrow_forwardShow work.arrow_forwardI waasnt sure about the sigma and pi bonds sentence. Would they not be broken because they are present in all bonds, no matter the type of IMF?arrow_forward
- Identify the strongest intermolecular force in the following molecule. :0: || Ha C-C O Hydrogen bonding O Dipole-dipole ineractions O London dispersion forces HICIHarrow_forwardBoron trifluoride readily combines with ammonia: BF3 + NH3 ® F3B-NH3. How does the hybridization of boron atom change in this reaction, if at all? From ___________ to ___________arrow_forwardWhy is water(H2O) more polar than NH3 and CH4?arrow_forward
- Please I want answer for this question by typing. Many Thanks DNA is a magical molecule that transmit genetic information from one generation to the other. Prepare a report on the importance of covalent bonding, hydrogen bonding and ionic interactions in determining the structure and stability of DNA molecules. (Word Limit 200)arrow_forwardPlease choose accurate and exact option i have only one chance so please be correct thanksarrow_forwardConsider how bond strength affects the speed of particles. Describe what happens to the kinetic energy of particles after a single bond is broken compared to a stronger double bond. You can answer this question with words, pictures, or graphs.arrow_forward
- please focus on this question 3 times i submitted it and three different answers, please make sure of ur answerarrow_forward* OWLV2 | Online teaching and lea 1.cengagenow.com/ilrn/takeAssignment/takeCovalentActvity do?locator =ESenment tal mistry P Algebra Google Keep = Economics - Googl. Norton d Destuns Clas, roam Video Libra Official Arkansas D. (References) If water is added to magnesium nitride, ammonia gas is produced when the mixture is heated. Mg, N2 (s) +3H20(1) → 3MgO(s) + 2NH3 (g) If 9.10 g of magnesium nitride is treated with water, what volume of ammonia gas would be collected at 21 °C and 738 mm Hg? Volume = Submit Answer Try Another Version 3 item attempts remaining pt Visited pt -pt pt DELLarrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY