
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Which of the following AB3 type molecules (T-shaped with trigonal bipyramidal electronic geometries) cannot exist, even in theory?
chlorine trifluoride (ClF3)
bromine trichloride (BrCl3)
fluorine triiodide (FI3)
iodine tribromide (IBr3)
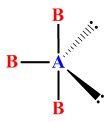
Transcribed Image Text:B-
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Element A has 6 valence electrons. Element B has 7 valence electrons. The central atom appears in one of the following periods: period 3, 4, 5, 6, or 7. They form a compound AB4. What is the molecular geometry of AB4?arrow_forwardAccording to valance bond theory, select which orbitals overlap to form a bond in the following molecule? HI 5s 5f 2s 5d 5p 3p 1s 4p 4s 3d 3s 2p 4d 4farrow_forwardWhat is the molecular geometry of the central S atom in the molecule SF4? tetrahedral trigonal bipyramid seesaw square planararrow_forward
- How many of the following molecules or ions are linear? NH3 OF2 HCN CO₂ NO₂ 0 0 1 O 2 3 4arrow_forwardAccording to molecular orbital theory, in the O2* ion, how many valence electrons are in antibonding molecular orbitals? 4arrow_forwardWhich of the following molecules or ions has a principal C4 axis? CF4 SF4 SiF4 XeF4arrow_forward
- 1. lewis structure of Vinyl magnesium bromide, CH2CHMgBr 2. assign the VSEPR model of the central atom3. Value of the corresponding theoretical angle4. classify 3 of the different bonds around the central atom as ionic or covalent5. draw the complete 3-D structure with respect to the central atom (s)6. draw the lewis structure for another mnolecule or species with a different atomic arrangementarrow_forwardWhat are the angles a and b in the actual molecule of which this is a Lewis structure? H H H C H a a = C b = ⁰ C H Note for advanced students: give the ideal angles, and don't worry about small differences from the ideal that might be caused by the fact that different electron groups may have slightly different sizes. H Xarrow_forwardWhich of the molecules are linear? BeCl, NO, XeF, SF,arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY