
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
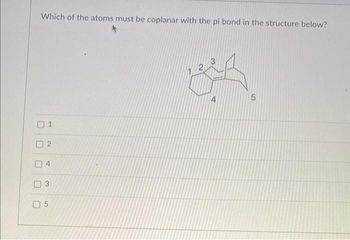
Transcribed Image Text:Which of the atoms must be coplanar with the pi bond in the structure below?
01
2
04
3
05
2
3
4
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Let Su, Tn, and VI be imaginary elements in the same column in the periodic table. Their relative electronegativities are Su > Tn > VI and their relative atomic radii are VI > Tn > Su. Which of the following anions would have the highest KÅ? HSuO3 HVIO3 HTnO3 All of these would be expected to have the same K₁.arrow_forwardWhich of the following is most likely to form a +2 ion? A) K B) Sc C) Al D) O E) Mgarrow_forwardI I I I I I I I I I I 4. Use Lewis Dot Diagrams to show the bonding between sodium and fluorine. I I I I I I I I I I I I I I Iarrow_forward
- Give the electron geometry (eg), molecular geometry (mg), and hybridization for Cl 3. eg = tetrahedral; mg = trigonal pyramidal; sp3 eg = trigonal planar; mg = trigonal planar; sp2 eg = octahedral; mg = tetrahedral, sp3 eg = tetrahedral; mg = linear; sp3 O eg trigonal pyramidal; mg = linear; sp3arrow_forwardHow many electron groups does the central atom of CH2O have according to VSEPR theory?arrow_forwardSpecify the correct answer. 12arrow_forward
- Which compound does not contain ionic bonds? a) NaNO3 b) Al₂O3 c) NiS2 d) HCI Which of the following molecules would be best described as polar? SiCl4 NH3 CO₂ BeCl2arrow_forwardIodine is an essential trace element in the human diet ( in most places, table salt comes with iodine as additive). Iodine deficiency causes goiter and thyroid problems. Is NaI an ionic or a covalent compound Give the steric numbers for the iodine atom and identify the geometries of the following ions containing iodine and oxygen IO3- IO65- IO4- Write the electronic configuration of the molecular ion I2+, what is the bond order?arrow_forwardWhat is the resonance form that describes the distribution of electrons in SeO2 ?arrow_forward
- 8. Draw the best Lewis structures of (a) AlF3 and (b) XeF4:arrow_forwardWhich of the following statements concerning the N₂O molecule is/are true? The atoms are bonded in the order NNO. You may select more than one, or none, of the responses. The central N atom is sp² hybridized. The structure of N2O is best represented as a hybrid of three equivalent resonance structures, There are two σ and two bonds. The N2O molecule is linear. The central N atom has a formal charge of +1. The N-N-O angle is 60°, The two N atoms have the same oxidation state.arrow_forwardGive the ground state electron configuration for O 2⁻. 1s22s22p6 1s22s22p4 1s22s22p3 1s22s22p5 1s22s22p2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY