
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
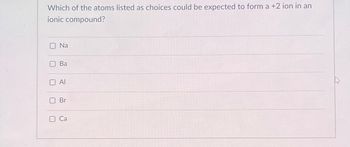
Transcribed Image Text:Which of the atoms listed as choices could be expected to form a +2 ion in an
ionic compound?
O Na
U
Ba
Al
Br
Ca
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Predict the chemical formula for the ionic compound formed by the ions Bal+ and C₂H3O2¯. tharrow_forwardHow many valence electrons does the following lewis dot structure represent? :Cl: O 7 LOarrow_forwardFor each of the following atoms, determine the minimum number of electrons the atom needs to gain, lose, or share to become stable (isoelectronic with a noble gas). N Mg S Siarrow_forward
- Using the electronegativity values given in the references section, show for the indicated bonds which atom acquires the partial negative charge. (To answer this question you may consult the table of electronegativity values in the table below.) H 2.1 1A 2A 3A 4A 5A 6A 7A Li Be B с N 0 F 1.0 1.5 2.0 2.5 3.0 3.5 4.0 Na Mg 8B Al Si P CI S 1.8 2.1 2.5 0.9 1.2 3B 4B 5B 6B 7B 1B 2B 1.5 3.0 K Ge As Se Br Ca Sc Ti V Cr Mn Fe Co Ni 1.0 1.3 1.5 1.6 1.6 1.5 1.8 1.8 1.8 Cu Zn Ga 1.9 .6 1.6 0.8 1.8 2.4 2.8 Rb Sr Y Zr 0.8 1.0 1.2 1.4 Nb Mo Te Ru Rh Pd 1.6 1.8 1.9 2.2 2.2 2.2 Ag Cd In Sn 1.9 1.7 Sb 1.7 1.8 1.9 2.1 Te I 2.5 TI Pb Bi Po Cs Ba La Hf 0.7 0.9 1.1 1.3 Ta W Re Os Ir Pt Au Hg 1.5 1.7 1.9 2.2 2.2 2.2 2.4 1.9 At 2.2 1.8 1.8 1.9 2.0 1.5-1.9 2.5-2.9 <1.0 1.0-1.4 2.0-2.4 3.0-4.0 If none of them bears the negative charge, choose none. (a) H3C-PH₂ (b) CH3P(H)-H Predict which indicated bond in each set is the more polar one. 8arrow_forwardWhen drawing bond line structures, which atom should NOT be labeled? oxygen O chlorine Onitrogen O boron carbonarrow_forwardWrite the empirical formula of at least four binary ionic compounds that could be formed from the following ions: Mg2+, Pb4+, I−, S2−arrow_forward
- Based on electronegativity difference, which contains bonds with the greatest covalent character? BeCl2 BaCl2 NaCl KClarrow_forwardComplete the table belowarrow_forwardWrite the empirical formula of at least four binary ionic compounds that could be formed from the following ions: 2+ Ca, Pb, Br, o² 4+arrow_forward
- Of the following elements, which has the highest electronegativity? Carbon Iron Nitrogen Bariumarrow_forwardWrite the empirical formula of at least four binary ionic compounds that could be formed from the following ions: 2+ 3+ Ca²+, Au³+, F, S²-arrow_forwardThe electronegativity value for N is 3.0 and that for O is 3.5. Based on these values, which of the following statements is TRUE about the compound NO? O NO is an ionic compound. O None of the above statements is true. O NO is a pure covalent compound. O NO is a polar covalent compound. O There is not enough enough information to determine the nature of NO.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY