
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Which of (a)-(d) shows the increasing order of basicity of compounds 1-4?
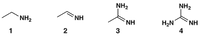
Transcribed Image Text:NH2
NH2
NH2
H.
NH
H2N
4
NH
1
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution with explanation needed. ..don't give Handwritten answerarrow_forwardPropose stepwise mechanisms for the following reactions.arrow_forwardb) 4. Rank the indicated protons in each of the given structures in order of decreasing acidity. Show the details for how you arrived at the answer. SHa HbN & OHCarrow_forward
- Within each structure, rank the indicated nitrogens by increasing basicity. Explain all (guide line: Increasing basicity. Begin by identifying the classification of the nitrogen.) (b) nicotine cyamemaine, an anti-psychotic drug Pentenyl cation Pentenyl anion (d) HN LY (a) C₂H₂CH-N-C,H, LSD CH₂0. Which of these belong to MO of Vin ,t-pentadienyl? Ponlenyl radical CH₂ H (e) CH-C) HN utabrine (anti-malarial) ipsapirone, an anxiolytic drug 8888 81818 - Which of these belong to HOMO in ,,-heptatrienyl radical? And how many electron/s it has? two e, one e two e, one e, - Construct a qualitative MO diagram for the following systems and discuss how the MOS are modified by addition of the substituent (each ¹ mark) a) CH=CH-CH=CH compared to CH=CH-CHO Acrolein (propenal) NET₂ NHỊCH, b) CH=CH-CN compared to CH=CH-COOH c) CH=CH-F compared to CH=CH-NH - Comparing FMO of the given compounds with each other while doing reaction with a nucleophile and electrophile a) CH=CH-CH=CH, compared to CH=CH-CHO…arrow_forwardCircle the compound in each of the following pairs that you would expect to be the stronger acid: (a) CH3CHClCO2H or CH2ClCH2CO2H (b) CH2FCO2H or CH2BrCO2Harrow_forwarde. List the given compounds in order of increasing acidity (lowest first). Give a reason for your answer. OH ELA H₂C H₂C ОН H₂C OH (i) (ii) (iii) f. A chemist has been asked to make the chemical shown below. Draw and name the one initial organic chemical used and devise a synthesis for the chemical below. Ensure you include all reagents and conditions. CH 3arrow_forward
- Nitromethane is quite acidic due to the stability of the nitromethane anion shown below. List two factors that afford stabilization of the nitromethane anion. H,CNO,arrow_forwardA reasonable synthesis for each of the following compoundsarrow_forward2 Rank the following compounds with increasing acidity: (1 = least acidic; 3 = most acidic) (a) HH (b) (c) (d) HoHarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY