
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
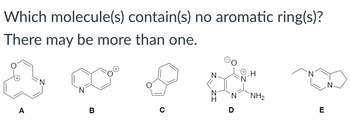
Transcribed Image Text:Which molecule(s) contain(s) no aromatic ring(s)?
There may be more than one.
NH2
A
B
C
D
E
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Draw all aromatic hydrocarbons that have molecular formula C3H19. For each compound, determine how many isomers of molecular formula CgH9Br would be formed if one H atom on the benzene ring were replaced by a Br atom.arrow_forward5. Which of these structures can be classified as aromatic and why? III IIarrow_forwardn of the following molecules could be considered aromatic? Assume all of the compounds are planar. T: Are these proper Lewis structures? ZI NH₂ N HBarrow_forward
- Identify the aromatic compounds. I II III IV I and II III and IV All of the answers are correct. O I, II, and IVarrow_forwardDr and name all possible aromatic compounds with the formula C8H9Br (There are 14)arrow_forwardDetermine if the following compounds are aromatic, non-aromatic, or anti-aromatic. Show a structure that helps to support your classification. This structure should not just redraw the compound; show lone pairs, resonance, and/or contributing π bonds as part of your explanation.arrow_forward
- From what we know today, what do the two Kekulé structures for benzene really represent? August Kekulé was the first person to propose a viable structure for benzene in 1865. Structures that can be separated at low enough temperatures (near absolute zero). Structures that are in a state of rapid equilibrium. O Structures that are in resonance. Structures that are conjugated trienes (--C=C--C%3DC--C3C--).arrow_forwardQUESTION 7 Which of the following is not true about anti-aromatic molecules? Anti-aromatic molecules must have 4N+2 pi electrons where N = 0, 1, 2, 3.... Anti-aromatic molecules must be cyclic Anti-aromatic molecules have to be planar Anti-Aromatic molecules are unstable compared to non-aromatic moleculesarrow_forwardFor each of the following compounds and ions,1. Draw a Lewis structure.2. Show the kinds of orbitals that overlap to form each bond.3. Give approximate bond angles around each atom except hydrogen.(a) [NH2]- (b) [CH2OH]+ (c) CH2“N¬CH3(d) CH3¬CH“CH2 (e) HC‚C¬CHO (f) H2N¬CH2¬CNarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
