
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
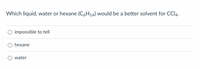
Transcribed Image Text:Which liquid, water or hexane (C6H14) would be a better solvent for CCI4.
impossible to tell
O hexane
water
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the correct answer and show the step by step solutionarrow_forwardThis graph shows how the vapor pressure of three liquids varies with temperature: 900 - 800 - 700- 600 500 400. 300 ethylbenzene pyridine isobutyl alcohol 200 100- +++H HHH HHHH 140 100 110 120 130 temperature, °C Use the graph to answer the following questions: Which liquid is the most volatile? most volatile: choose one Which is the least volatile? least volatile: choose one ethylbenzene: What is the normal boiling point of each liquid? Note: your answer must be within 1°C of the exact answer to be graded correct. pyridine: isobutyl alcohol: Suppose a beaker of pyridine is put inside a sealed tank containing pyridine gas at 112. degree C and 692. torr. After ten minutes, will there be more liquid in the beaker, less liquid, or the same amount? O less the same vapor pressure, torrarrow_forwardIn the supermarket why do they spray their produce with water on a regular basis?arrow_forward
- Please answer as soon as possibly and I will leave a like.arrow_forwardCan water stay liquid below zero degrees Celsius?arrow_forwardChoose the pair of substances that are most likely to form a homogeneous solution.Group of answer choices NH2CH3 and CH4 None of the pairs above will form a homogeneous solution. NF3 and SO2 CI4 and SI2 CO and C5H12arrow_forward
- n-pentane is a hydrocarbon that is a straight chain of 5carbons. 2 methyl butane is a straight chain of carbons with one side chain of 1 carbon. They both have the same molecular mass. Which one boils at a higher temperature No way t know since the density was not given. n-pentane They both boil at the same temperature. 2-methyl butanearrow_forwardMethanol and propan-2-ol form a homogenous solution. Which type(s) of forces are involved? dispersion force, dipole-dipole, hydrogen bonding dispersion force, dipole-dipole, hydrogen bonding, ion-dipole force dispersion force, dipole-dipole force dispersion forcearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY