
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
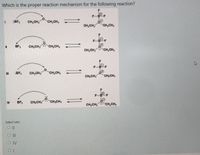
Transcribed Image Text:Which is the proper reaction mechanism for the following reaction?
F
OT
F-BF
:BF3
CH2CH3
CH2CH3
CH,CH,
CH,CH3
F
F-BF
BF3
CH,CH,
CH,CH,
CH,CH, CH,CH,
F-B-F
:BF3
CH,CH,
CH,CH3
CH2CH3
CH2CH3
FB F
IV
BF3
CH2CH3
CH2CH3
CH2CH, CH,CH3
Select one:
O |I
O IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following is an intermediate in the reaction below? A) H3t H₂CH H B (Sia)₂ ☆ó B) H₂ (~= UH CH₂CH H H E) H₂E NOH 1. (Sia) 2BH THF 2. NaOH, H₂ de. H D) H3 (1 нarrow_forward14. All of the following involve a multi-step mechanism except A) B) C) D) nd O id cuc nd nd HCI CH3COOH H₂O, H Br2 Cl X t Br OH Brarrow_forwardagents Which set of reagents will accomplish this reactions? он HO,C CO,H HO,C čO,H он А MCPBA, aqueous NaOH, then H3O* H2O2 in methanol, I2, then H30* KMNO4, aqueous NaOH, then H3O* H3O*, 80 ° C, 4 hours Prof. Friedric CHM 2270 01-Dec-20arrow_forward
- Which of the reactions shown below are correct? & OH oxo H₂O* CO₂H CO₂Et CO₂Et NaOCH₂CH3 HOCH₂CH₂ NaOCH₂CH3 HOCH₂CH3 요 CO₂Et H₂C CH3CO₂Et + CHCO₂Et H₂Carrow_forward7. Predict the product and draw the 2-step mechanism. 1. ROH attacks ZnCl2, giving the O-atom a (+) charge and Zn a (-). The ZnCl, does not lose a CI- ion, it's simply attached to the OH group. 2. Then a CH ion attacks and kicks off the better leaving group (Sn2). ZnCl2 HOarrow_forwardWhat is the major product in step B of the reaction sequence shown? O CH₂CH₂CI AICI3 H₂C. S CH3 NBS NH₂ A Br CH3 NBS CH₂OOCH, BH₂ CH3 B NaOCH, CH,OH C CH₂OOCH3 Darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY