
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
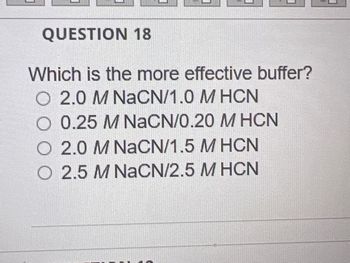
Transcribed Image Text:QUESTION 18
Which is the more effective buffer?
2.0 M NaCN/1.0 MHCN
0.25 M NaCN/0.20 M HCN
O 2.0 M NaCN/1.5 M HCN
O 2.5 M NaCN/2.5 M HCN
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- You wish to preparc a buffer that can resist large additions of basc, but not large additions of acid. Which buffer system would be most appropriate? a) 0.2M HF/2.0M NaF b) 2.0M HF/2.0M NaF c) 2.0M HF/0.2M NaF d) 0.2M HF/0.2M NaFarrow_forwardWhat is a buffer? Choose 2. a solution that resists changes in pH a solution that neutralizes a basic solution a weak acid-base conjugate pair a solution that neutralizes an acidic solution a strong acid-base conjugate pairarrow_forwardHow many of the following three pairs would make an effective buffer?NaNO 2/NaOHNaF/NaOHNH 4Cl/NH 3arrow_forward
- Which pair of compounds will form a buffer in aqueous solution? HCNHCN and HClHCl NaCNNaCN and KCNKCN HClHCl and NaOHNaOH HCNHCN and NaCNNaCN NaCNNaCN and NaOHNaOH HClHCl and NaClarrow_forwardA buffer pair consists of a conjugate weak acid0weak base pair that will resist a change in pH from the addition of a strong acid or base. Decide whether each pair below is a good example of a buffer.a. HCN and NaCN Good Buffer or Not a Bufferb. H2CO3 and Na2CO3 Good Buffer or Not a Bufferc. H2SO4 and NaHSO4 Good Buffer or Not a Bufferarrow_forwardWhich of the following would destroy a buffer containing 0.134 moles of weak acid HA and 0.268 moles of conjugate base A -? 0.134 mol of HCl 0.186 mol of HCl 0.0670 mol of NaOH 0.0447 mol of NaOH 0.268 mol of HClarrow_forward
- Select the combination that results in the most effective buffer? O 0.800 M HNO2; 0.799 M NANO2 O 0.800 M HCOOH; 0.100 M KCOOH 0.700 M HNO3; 0.599 M NaNO3 O 0.100 M HBr; 0.899 M NH4Brarrow_forwardWhich of the following acid/base pairs can be used to prepare a buffer? a) NaH2PO4/NaHCO3b) HCl/NaOHc) NH4+/NH3d) HNO3/NaNO3arrow_forwardWhich of the following solutions will be buffers? Explain your answer in each case. a) 0.10 M KCN mixed with 0.10 M HCN b) 1.0 M NaOH mixed with 1.0 M NaClarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY