
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
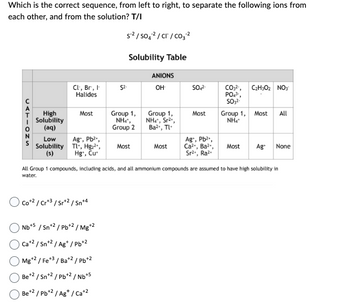
Transcribed Image Text:Which is the correct sequence, from left to right, to separate the following ions from
each other, and from the solution? T/I
CATIONS
High
Solubility
(aq)
Low
Solubility
(s)
Cl, Br, I
Halides
Most
Ag+, Pb²+,
Tl+, Hg₂²+,
Hg, Cu
Co+2 / Cr+3/Sr+2 / Sn+4
S²/SO4²/CT/CO₂ ²
Nb+5 / Sn+2/Pb+2 / Mg+2
Ca+² / Sn+² / Ag*/Pb+2
Mg+2 / Fe+3 / Ba+2/Pb+2
Be +2 / Sn+2/Pb+2/Nb+5
Be+2 / Pb+² / Ag+ / Ca+2
Solubility Table
Group 1,
Nhì,
Group 2
Most
ANIONS
OH
Group 1,
NH4*, Sr²*,
Ba²+, Tl
Most
SO4²-
Most
Ag+, Pb²+,
Ca²+, Ba²+,
Sr²., Ra².
CO₂²¹, C₂H302 NO3
PO4³-,
SO3²-
Group 1,
NH4*
Most All
All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in
water.
Most Ag+ None
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following anions will separate Ag* from Pb** by precipitating one of them, leaving the other in solution? (A) CATIONS High Solubility (aq) Low Solubility (s) OOH™ O cr O C₂H30₂ 3- PO4³- Cl, Br, I Halides SO4²- Most Ag+, Pb²+, Tl+, Hg₂²+, Hg, Cu Solubility Table $2. Group 1, Nhì, Group 2 Most ANIONS OH Group 1, NH4*, Sr²⁰, Ba²+, Tl. Most SO4²- Most Ag+, Pb²+, Ca²+, Ba²+, Sr², Ra² CO32, C₂H3O2 NO3 PO4³-, SO3²- Group 1, NH4 Most All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in water. Most All Ag+ Nonearrow_forwardIf 316.0 mg of AgBr is completely dissolved in 144.0 mL of 0.12 M NH3, what is the equilibrium concentration of Ag+(aq) in the final solution? Kf[Ag(NH3)2+] = 1.7E7 Include 3 significant figuresarrow_forward4. A solution prepared to be initially 1 M in NH3 and 0.5 M in HCl is (Kb for NH3 = 1.8 x 10¯5): (A) a solution with a pH less than 7 that is not a buffer solution (B) a buffer solution with a pH between 4 and 7 (C) a buffer solution with a pH between 7 and 10 (D) a solution with a pH greater than 7 that is not a buffer solution (E) a solution with a pH of 7arrow_forward
- (a) Write the net ionic equation for the reaction that occurswhen a solution of hydrochloric acid (HCl) is mixed with asolution of sodium formate (NaCHO2). (b) Calculate theequilibrium constant for this reaction. (c) Calculate theequilibrium concentrations of Na+, Cl-, H+, CHO2-, andHCHO2 when 50.0 mL of 0.15 M HCl is mixed with 50.0 mLof 0.15 M NaCHO2.arrow_forwardGive me a clear handwritten answer with explanation...arrow_forwardyou want to separate Ni2+ and Mn2+ by precipitating the corresponding sulphidesfrom each other. What pH value must you set so that one of the twometals is precipitated quantitatively (concentration in the solution less than/equal to 10/-5 mol/l)is precipitated while the other is still in solution? Ksp (NiS): 10^-21, Kps (MnS): 10~15; Ks (H2S): 10 ~- 20,Saturation concentration of H2S in water: 0.1 mol/larrow_forward
- Give detailed Solution with explanation (don't give Handwritten answerarrow_forwardWhat minimum volume of 0.09621 M AgNO3 willbe needed to assure an excess of silver ion in the titration of: (a) an impure NaCl sample that weighs 0.2513 g?(b) a 0.3462-g sample that is 74.52% (w/w) ZnCl2?(c) 25.00 mL of 0.01907 M AlCl3?arrow_forwardWhich of the following will be insoluble with sulfates but soluble with halides? CATIONS High Solubility (aq) Low Solubility (s) Cl, Br, I Halides Most Ag+, Pb²+, Tl+, Hg²+, Hg', Cu OCa²+, Sr²+, Ba²+ OPb²+, Hg²+, Ag+ Sr²+, Ba²+, Pb²+ 52. OCa²+, Hg2+, Ag+ O cut Solubility Table Group 1, NH₁", Group 2 Most ANIONS OH Group 1, Nhĩ, Srz Ba²+, Tl Most SO4²- Most Ag", Pb²+, Ca²+, Ba²+, Sr²+, Raz CO₂², PO¹, SO3² Group 1, NH4 C₂H₂O₂ NO All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in water. Most All Most Ag+ Nonearrow_forward
- If 316.0 mg of AgBr is completely dissolved in 144.0 mL of 0.12 M NH3, what is the equilibrium concentration of Ag+(aq) in the final solution? Kf[Ag(NH3)2+] = 1.7E7 Include 3 significant figures THE ANSWER IS NOT 0.387arrow_forwardThe literature Ksp value for Mg(OH)2 is 1.8×10¬11. (a) Calculate the theoretical [OH ] in a saturated solution of Mg(OH)2. 4.0 3.3e-4 (b) What volume of 0.00177 M HCI would be required to neutralize the OH- in 100.0 mL saturated Mg(OH)2 solution? 4.0 mLarrow_forwardA solution may contain any or all of the following cations: Ag+, Pb2+, Ba2+, Ni2+ in an UNKNOWN mixture. HCl is added to the solution and a precipitate forms. That precipitate is removed and NH3 (aq) is added to the supernatant until the solution is basic, no solid forms. After making the supernatant basic, K2CrO4 is then added and a precipitate forms. What cations (Ag+, Pb2+, Ba2+, Ni2+)are present in this unknown sample (This is chemistry and not a writing question)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY