
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Solve correctly please.
Q.Which is an anode?
a) copper metal
b) copper (II) ion
c) zinc metal
d) zinc ion
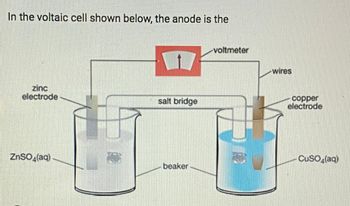
Transcribed Image Text:In the voltaic cell shown below, the anode is the
zinc
electrode
ZnSO4(aq)
salt bridge
beaker
voltmeter
1
wires
copper
electrode
CuSO (aq)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hydrogen acts as a reducing agent by _____ giving electrons only taking oxygen only taking hydrogen taking oxygen and giving electronsarrow_forwardSelect a statement that best describes the oxidation process. A) In the oxidation process all elements change oxidation state. B) In the oxidation process all elements experience an increase in oxidation state. C) In the oxidation process some elements change their oxidation state. D) In the oxidation process some elements experience oxidation state increase. E) In the oxidation process only oxygen increases its oxidation state.arrow_forward2. Consider the dichromate polyatomic ion, what is the oxidation number of chromium, Cr?arrow_forward
- a) Write a balanced chemical equation describing the oxidation of chlorine gas by the copper(III) ion to form the chlorate ion and copper(II) in an acidic aqueous solution. Use the smallest whole-number coefficients possible. b) How many electrons are transferred in the redox reaction?arrow_forwardWhat is the oxidation number of nitrogen in the nitrate ion? a)+1 b)+6 c)-1 d)+5arrow_forwardWhat is a battery? Why is distilled water a weaker conductor than tap water? Why does solid sodium chloride act as a non-electrolyte while an aqueous NaCl solution acts as a strong electrolyte? 4. Is it possible to have an electrical current that consists of something other than electrons?arrow_forward
- Question is: For each of the conbinations of metal and solution listed below, predict if a single displacement reaction will occur. If a reaction will occur write a blanced chemical equation for each example. If no reaction is expected, write no reaction. Please complete all and needed asap!!!!arrow_forwardD) Oxidation-Reduction Reactions (Single Replacement) 7) Hydrogen is released when aluminum reacts with hydrochloric acid. 8) Magnesium reacts with silver nitrate solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY