
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:b.
The C-2 atom in 2-phosphoglycerate is not acidic in solution, i.e. it does not have a tendency to lose
its proton (colored blue above). However, in the active site of enolase, C-2 has a lowered pKa and is acidic
enough that Lys45 can extract its proton. Which feature of the enzyme active site do you expect to increase
the acidity of C-2?
(Hint: The transfer of the double bond on to the C-2 atom is directly linked to and stabilized by the ability of
the carboxyl group of 2-PG to become -2 charged.)
C.
Using acid-base catalysis terminology, state the roles for Glu211 and Lys345 in step 1. In other words,
what do the two side chains serve as?
To fulfill their roles, do you expect Glu211 and Lys345 to have modified pka's as compared to those of
free Glu and Lys side chains? If so, do you expect elevated or decreased pKa's for these residues within
d.
the context of the active site of enolase?
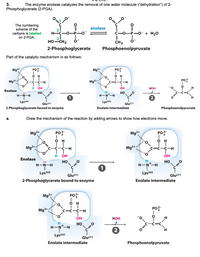
Transcribed Image Text:3.
The enzyme enolase catalyzes the removal of one water molecule ("dehydration") of 2-
Phosphoglycerate (2-PGA).
The numbering
scheme of the
enolase
carbons is labeled
HC-0-P-O
+ H20
on 2-PGA.
||
CH2
HO-CH2
2-Phosphoglycerate
Phosphoenolpyruvate
Part of the catalytic mechanism is as follows:
Mg2+
PO?-
Mg?+
PO?
'o.
Ó H
Mg?+
Mg²+;
PO?
:-C-H
C=C-C-H
OH
OH
Нон
Enolase
но
но
H-N*-H
Lys345
Lys345
Glu211
Glu211
2-Phosphoglycerate bound to enzyme
Enolate intermediate
Phosphoenolpyruvate
а.
Draw the mechanism of the reaction by adding arrows to show how electrons move.
Mg2+
PO?-
Mg2+
po
PO
H
H
Mg2+
С -с-с-н
Mg2+
C=c-C-H
H
OH
OH
Enolase
H.
H-N-H
но
но
..
H-N-H
Lys345
Lys345
Glu211
Glu211
2-Phosphoglycerate bound to enzyme
Enolate intermediate
Mg?+
PO
O.
он
Mg2+:
PO?
c=C-C-H
OH
HOH
H
но
C=C
H-N*-H
Lys345
Glu211
Enolate intermediate
Phosphoenolpyruvate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- What are some of the interactions made between ATP and the kinase? Describe bonds that the protein makes with each of the following: the phosphate groups the sugar the adenine basearrow_forwardWhy are most enzymes larger than the substrates on which they act?arrow_forwardThere are several types of enzyme regulators which can either be positive ("turn on") or negative ("turn off") regulators. Why would you ever want to "turn off" an enzyme?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON