
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can someone explain why this is the answer? thank you
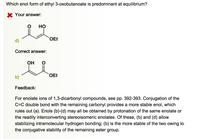
Transcribed Image Text:Which enol form of ethyl 3-oxobutanoate is predominant at equilibrium?
K Your answer:
но
OEt
d)
Correct answer:
Он
OEt
b)
Feedback:
For enolate ions of 1,3-dicarbonyl compounds, see pp. 392-393. Conjugation of the
C=C double bond with the remaining carbonyl provides a more stable enol, which
rules out (a). Enols (b)-(d) may all be obtained by protonation of the same enolate or
the readily interconverting stereoisomeric enolates. Of these, (b) and (d) allow
stabilizing intramolecular hydrogen bonding; (b) is the more stable of the two owing to
the conjugative stability of the remaining ester group.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the following acid - base reactions, a) Draw Lewis structures of the reactants and the products. b) Determine which species are acting as electrophiles (acids) and which are acting as nucleophiles (bases). c) Use the curved - arrow formalism to show the movement of electron pairs in these reactions and the imaginary movement in the resonance hybrids of the products. d) Indicate which reactions are best termed Brønsted-Lowry acid - base reactions i. CH3CHO + HCI-- > CH3CH2O + + Cl- ii. CH3CHO + OH- - - > CH3CO-(OH) Harrow_forwardPlease send me the question in 30 minutes it's very urgent plzarrow_forwardWhy is HCl so acidic? For example, why is H-Cl more acidic than H-S-CH3? Isn't the conjugate base's negative charge more stabilized with a sulfur and extra methyl group (by the induction effect)? While Cl is more electronegative than Sulfur, I would assume Cl being alone as its own ion would make it less stable.arrow_forward
- a) b) What is the conjugate acid of NH3? What is the conjugate base of H2O?arrow_forwardThe alkyl halide shown below in line-bond structure is the product of ethene hydrohalogenation. Choose the model(s) that correspond to this conformation. с D A B H H H H A O -Harrow_forwardI need help with this question. Can you help me to show the work like step by steparrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY