
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:Q
F4
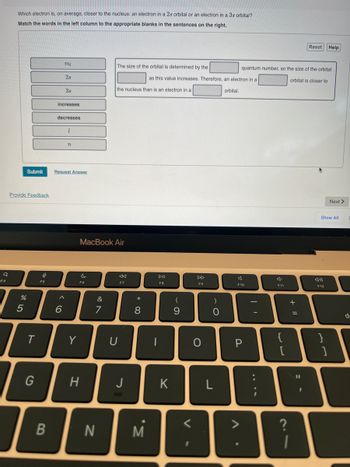
Which electron is, on average, closer to the nucleus: an electron in a 2s orbital or an electron in a 3s orbital?
Match the words in the left column to the appropriate blanks in the sentences on the right.
Submit
Provide Feedback
%
5
T
G
9
F5
B
mi
2s
38
increases
6
decreases
1
n
Request Answer
Y
c
MacBook Air
F6
H
&
7
N
The size of the orbital is determined by the
the nucleus than is an electron in a
<
F7
* 00
J
8
이
U
M
as this value increases. Therefore, an electron in a
DII
F8
K
(
9
F9
V
O
ş
)
O
L
orbital.
quantum number, so the size of the orbital
orbital is closer to
F10
P
A
F11
لال
{
?
+ 11
Reset Help
=
Show All
»)
F12
Next >
}
d
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Questions: Quantum Theory, Atomic Structure, and Periodicity Due in 26 minutes 9/15 answered HQ7.43 Homework • Unanswered Rank the following from highest ionization energy (at top) to lowest ionization energy (at bottom). Drag and drop options into correct order and submit. For keyboard navigation... SHOW MORE v Aluminium Chlorine Sodium Argon Phosphorus Unanswered • 2 attempts left hulu IIarrow_forwardWhich transition emits the highest energy photons? -- Multiple choice A → B A → C B → C B → A C → A C → Barrow_forwardt ot pt pt pt 1 pt 1 pt 1 pt 1 pt at 1 pt 3 1 pt 1 pt 1 pt What is the maximum number of orbitals that can be identified by the following set of quantum numbers? Note that in cases where the set of quantum numbers is not "allowed," the answer will be zero. a. n = 2, l = 2, me = 0 orbital(s) 1 pt gnment b. n = 4 Submit Answer orbital(s) Try Another Version [References] 10 item attempts remaining Cengage Learning Cengage Technical Support the new Terms and Conditions. MacBook Pro Previous Next Save and Exitarrow_forward
- What is the smallest possible value of the principal quantum number n for a p electron? TOCAMLAGI Jobinel 11 = Continue 0 1-95 S/US-1 S/... Closed road X 3 Q Search S 2023 McGraw Hill LLCarrow_forward6ab 10mins asaparrow_forwardBro and pro expert Hand written solution is not allowed. Give proper explanation of the correct option and proper explanation of the incorrect options will upvote.arrow_forward
- Part A Cs Express your answer in condensed form in the order of orbital filling as a string without blank space between orbitals. For example, He 2s 2p should be entered as [He]2s^22p^2. [Xe]6s^1 Previous Answers V Correct Our first step is to write the noble-gas core We do this by locating cesium, element 55, in the periodic table. We then move backward to the nearest noble gas, which is Xe, element 54 Thus, the noble-gas core is Xe. Next, we trace the path in order of incre Moving from Xe to Cs, element 55, we find ourselves in period six of the s block. Knowing the block and the period identifies the subshell in which we begin placing outer electrons, 6s. As we move through the s block, we add one electron, giving us Xe 6s Рart B Ni Express your answer in condensed form in the order of orbital filling as a string without blank space between orbitals. For example, Hel2s 2p should be entered as [He]2s^22p 2. Submit Request Answer Part C Se Express your answer in condensed form in the…arrow_forwardesc Page 1: 1 4 ! 7 10 13 16 2 19 5 ✓ 8 9 11 12 HAB 14 15 17 18 11 20 21 S 22 23 24 25 26 27 D F1 3 6 ! F2 Answer the following question by matching the numbers with the descriptions. Numbers may be used more than once. Not all the numbers will be used. 1. 1 2. 2 3. 3 4. 4 5. 5 6. 6 The number of orbitals in a 4d subshell or sublevel 7. 7 The number of electrons in a full 5p orbital. 8. 8 The number of electrons in 9. 9 a full 3d subshell or sublevel 10. 10 80 F3 F4 F5 MacBook Air F6 AA F7 DII F8 A F9 F10 F11arrow_forwardWhen writing the ground-state electron configuration of a many-electron atom, three main rules must be followed: . The aufbau principle: Electrons are added to the lowest energy orbitals available. . The Pauli exclusion principle: No two electrons in an atom can have the same set of four quantum numbers (n. l. me, and m₂). • Hund's rule: For degenerate orbitals, the lowest energy state is attained when the number of electrons with the same spin is maximized. So for a degenerate set of orbitals, one electron goes into each orbital until all the orbitals of the subshell are half-filled. Once all the orbitals of the subshell are half-filled the pairing of electrons can take place. Note that aufbau is the German word for "building up." ▾ Part A Classify each orbital diagram for ground-state electron configurations by the rule or principle it violates. Drag the appropriate items to their respective bins. View Available Hint(s) Aufbau violation 3p 111 3p 111 3p 11 3s 11 45 1 Hund violation…arrow_forward
- Hi I’m confused here, which is the right answer?arrow_forwardPls help ASAP. Pls do both of the asked parts.arrow_forwardComplete the table describing orbitals in the n=4 energy level: l Possible ml values Orbital name(s) 0 0 4s (1 orbital) 1 -1, 0, +1 ____ (3 orbitals) 2 ____ (__ orbitals) 3 ____ (__ orbitals) Total number of orbitals = ________ Maximum number of electrons in the n = 4 energy level = __________arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY