
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
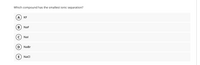
Transcribed Image Text:Which compound has the smallest ionic separation?
A
KE
В
NaF
Nal
D
NaBr
E
NaCI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which ion has twenty-six electrons? Group of answer choices Ni2+ Fe2+ Cr2+ Cu2+arrow_forwardList the compounds in order of decreasing covalence and explain why: HI, HCl, HF, HBrarrow_forward1. Put the electron suborbitals into order of energy, with the highest energy at the top and lowest energy at the bottom 2. Which of the following solids is most likely to contain only metallic bonds?arrow_forward
- I need help with questions 12-13? Could you explain which option is correct for each question?arrow_forwardWhat would be the observation following mixing BaCl2 and Pb(NO3)2?arrow_forwardSharing ngs ols > The electronegativity values of representative elements in group 1A (1) to group 7A (17) 1 2 Group Group 1A 2A Li 1.0 Na 0.9 K 0.8 Rb 0.8 Cs 0.7 Be 1.5 Mg 1.2 Ca 1.0 Sr 1.0 Ba 0.9 H 2.1 13 14 15 16 17 Group Group Group Group Group 3A 4A 5A 6A 7A B 2.0 C 2.5 Al 1.5 1.8 Si P 2.1 ΤΙ 1.8 Ga Ge As 1.6 1.8 2.0 N 3.0 In Sn 1.7 Pb 1.9 O 3.5 Sb Te 1.8 1.9 2.1 Bi 1.9 S 2.5 Se 2.4 Po 2.0 F 4.0 Cl 3.0 Br 2.8 I 2.5 At 2.1 18 Group 8A Part A Show the dipole arrow for each of the following bonds. Drag the appropriate labels to their respective targets. 00 P-O S-CI P Pearson N-F Submit Previous Answers Request Answer X Incorrect; Try Again; 4 attempts remaining P-F Reset Help O-CI Torms of Use | Privacy Policy | Permissions | Contact Us |arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY