
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
30
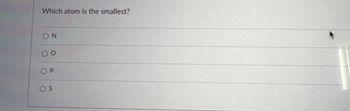
Transcribed Image Text:Which atom is the smallest?
ΟΝ
0 0
OP
OS
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- -> Ô https://app.101edu.co Question 25 of 41 A patient is required to take an IV drug for cancer treatment. The required dosage is 5.0 mg drug per Ib patient body weight every day. Each of the bags of the drug contains 250mg drug. If you are treating a 150 Ib patient, how many bags of the drug are needed each day? 150 Ib patient 750 mg drug 5.0 mg drug 1 STARTING AMOUNT 150 lb patient 5.0 mg drug x * 750 mg drug 1 ADD FACTOR DELETE ANSWER RESET 5.0 0.020 0.12 50.0 30.0 7.5 x 10 750 0.33 250 3.0 1 10.0 8.3 150 0.60 1250 mg drug/bag mg drug bags Ib patient mg drug/lb patient LO O F1 F2 Prisc F3 F4 FS F6 F7 FO FO F10 F11 F12 %23 & 1 6 Q W Y U 1O P S. F J K 44R の 山arrow_forwardYou have a sample of 121.5 g of 26. How many moles of 26 are in the sample? Show your work. Be sure each step is written out clearly. H E E A A Normal + Enter your answer here = = = © BIUS √x 0 0 T X₂ X² 400 worarrow_forwardX, Mass of sample (g) = 0.7 Y, Mass of benzoic acid (g) = 0.3 Z, Mass of pellet (g) = 0.9 calculate the follwing below information Mass of sample in pellet = Mass of benzoic acid in pellet =arrow_forward
- acrose costs $1.05 per pound (lb). If a bottle has 2.00 kg of sucrose in it, how much would you pay for a se of sucrose containing 12 bottles? (1 lb-453.59 g) Hint: start with number of bottles A) $55.56 B) $50.94 $61.89 D) $58.61 Shatom moulearrow_forward16. Chloral hydrate, a sedative and sleep-inducing drug, is available as a solution labeled 10.0 gr/fluidram. What volume in milliliters should be administered to a patient who is meant to receive 7.5 grains per dose? (1 gr = 64.8 mg; 1 fluid ram 3.72 mL) d of 400 feearrow_forwardA prescription for allergies reads one dose of Promethazine 0.100 g, three times a day. The pharmacist dispenses 25 mg Promethazine tablets. How many tablets will need to be administered per dose? What dose of Promethazine will this patient need to take in 24 hours?arrow_forward
- A sample of metal weighing 1.4 g is combined with oxygen; the metal oxide weighs 33.5 g. The mass percent of oxygen in the compound is _____.arrow_forward=2591470&isprv3&drc%3D0&.gi=247580 How many grams of H2SO4 are present in 4.0*102 formula units of H2S04? Round your answer to 1 decimal place. Your Answer: Answer units D Add attachments to support your work Previous Page Next Page Page 1 of 6arrow_forwardThe dosage of quinine when a 145−lb adult takes a 200.−mg tablet is equivalent to _____ μg drug per kg of body weight.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY