
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
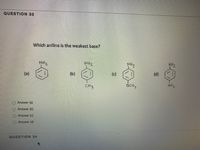
Transcribed Image Text:QUESTION 33
Which aniline is the weakest base?
NH2
NH2
NHZ
NH2
(c)
(d)
(a)
(b)
OCH,
NO2
CHz
O Answer (a)
Answer (b)
O Answer (c)
O Answer (d)
QUESTION 34
O O O 0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardRank the solutions below in order of increasing acidity. Rank the solutions below in order of increasing acidity. Correct Answer List Highest acidity 0.01 M H2SO4 0.01 M CH3COOH 3 M NH3 0.1 M NaOH 0.1 M HClarrow_forwardWhich of the following is a weak base? a) CH3OH b) HCOOH c) LiOH d) CH3NH2 e) Ca(OH)2arrow_forward
- 20) associated with allergies, is shown below. Which N atom is the most basic and which H atom is the most acidic? Histamine, one of the compounds responsible for the symptoms Most Basic N Most Acidic H II (a) II (b) II H (c) (d) Y (e) Y N Narrow_forwardWhich of the following acids is the strongest acid? Group of answer choices HI HBr Acetic acid HF Which of the following bases is the WEAKEST? The base is follow by its Kb value.a) C2H5NH2, 1.7x10-9 b) (CH3CH2)3N, 5.2x10-4 c) NH3, 1.8x10-5 d) HOCH2CH2NH2, 3.2x10-5arrow_forward4 O 1.5 § O Base N Н HIC-H N=C-C-H + Base ин acid Н H-Z + Base + X : + H H-CI acric O O=S=O O-S-C Ocific Oz: Вазе acidic H - N=C- acid 3 chearrow_forward
- Among the following compounds select a match for each category A OH Highest boiling point Strongest acid 2nd strongest acid HO The worst smelling B OH [Choose ] [Choose ] CDBA A [Choose ] [Choose ] SH Darrow_forwardpick the best base. if none of the above are correct, please give correct base and a brief explainationarrow_forwardBromfenac is a nonsteroidal anti-inflammatory drug. Select the modification that will make Bromfenac a weaker base. Bromfenac NH2 OH Br NH OH Br oror NH2 OH Br NH2 OH Brarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY