
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
PLEASE HELP ASAP.
PHYSICS ALGEBRA
With exact decimals!
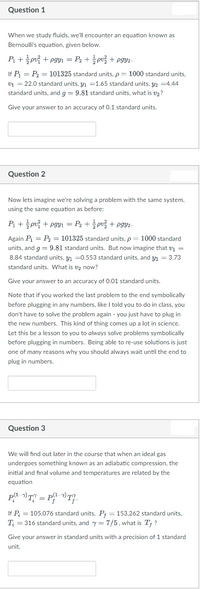
Transcribed Image Text:Question 1
When we study fluids, we'll encounter an equation known as
Bernoulli's equation, given below.
P + pv + pgYı = P2 + pv + pgy2-
If P = P2 = 101325 standard units, p = 1000 standard units,
v1 = 22.0 standard units, Y1 =1.65 standard units, 42 =4.44
standard units, and g = 9.81 standard units, what is v2?
Give your answer to an accuracy of 0.1 standard units,
Question 2
Now lets imagine we're solving a problem with the same system,
using the same equation as before:
P+ pui + pgyi = Pz + 슬pu을 + pgy.-
Again P = P2 = 101325 standard units, p = 1000 standard
units, and g = 9.81 standard units. But now imagine that v1 =
8.84 standard units, y1 =0.553 standard units, and y2 = 3.73
standard units. What is v2 now?
Give your answer to an accuracy of 0.01 standard units.
Note that if you worked the last problem to the end symbolically
before plugging in any numbers, like I told you to do in class, you
don't have to solve the problem again - you just have to plug in
the new numbers. This kind of thing comes up a lot in science.
Let this be a lesson to you to always solve problems symbolically
before plugging in numbers. Being able to re-use solutions is just
one of many reasons why you should always wait until the end to
plug in numbers.
Question 3
We will find out later in the course that when an ideal gas
undergoes something known as an adiabatic compression, the
initial and final volume and temperatures are related by the
equation
-
If P = 105,076 standard units, P; = 153,262 standard units,
T; = 316 standard units, and y = 7/5, what is T† ?
Give your answer in standard units with a precision of 1 standard
unit.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Not an essay, i need to solve this math problemarrow_forwardhow it become 0.829? if i multiply the numbers it will give me 5096?arrow_forwardState the number of significant digits and the implied precision of the given number. The number 1.928×10^4 has........ significant digits (type a whole number) B. What is the implied precision of the number 1.928×10^4 s? A. The number is precise to the nearest hundred seconds B. The number is precise to the nearest second. C. The number is precise to the nearest ten secondsarrow_forward
- For this question, can you explain how we get the integral from dFz to Fz. Can you also explain how im supposed to know which proportion to use? In some similar question we use proportions such as dQ/dl and in other question we use dz/d theta. How do i know which proportion to use for the question?arrow_forwardhat number w.ww The word "units" refers to the corresponding quantity of something. For example, 2 is a number; 2 seconds is a physical quantity that has the units of second. In physics problems, are usually not looking for a number; we are looking for a need to also know the units. If you do not include units with the answer, you will not receive physical quantity, which means we full credit. Physicists are very nitpicky about this! 8. You may have learned the formula distance speed x time. How long would it take in seconds to travel a distance of 2000 meters if you are traveling at a speed of 25 meters per second? (note: your answer must include the word "seconds" or the abbreviation "s" after your number...remember your units! \Sxt t1475 2000 25tt 2000 -25t 1975-t 9. How long would it take in minutes to travel a distance of 2000 meters if you are traveling at a speed of 50 meters per second? S X t e1950 2o00 Sot 2000-50-t 1950 10. How far does sound travel in 1 minute? Put your…arrow_forwardThe answer is 30 but I don't know how it arrived at that answer! Please explainarrow_forward
- a. 2πR2+2πRh2πR2+2πRh b. 2πR2+2πh2πR2+2πh c. πR2hπR2h d. πD2+πDhπD2+πDh e. 2πR+2πRharrow_forwardProblem 1: Convert to Sl and write in scientific notation: a) 55 mph (miles per hour) to m/s b) 6.0 gallons to liters c) 1.282 light-seconds to m d) 1 acre to m²arrow_forwardPhysics Please help me with this question It was from a Lab that we have to understand but im unsure how to answer this.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON