
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Answer please
Transcribed Image Text:Question 16.b of 20
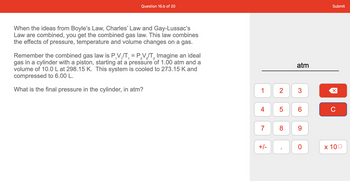
When the ideas from Boyle's Law, Charles' Law and Gay-Lussac's
Law are combined, you get the combined gas law. This law combines
the effects of pressure, temperature and volume changes on a gas.
1 1 1
2 2 2
Remember the combined gas law is P.V₁/T₁ = P_V₂/T₂ Imagine an ideal
gas in a cylinder with a piston, starting at a pressure of 1.00 atm and a
volume of 10.0 L at 298.15 K. This system is cooled to 273.15 K and
compressed to 6.00 L.
What is the final pressure in the cylinder, in atm?
1
4
7
+/-
2 3
LO
5
atm
8
CO
6
9
0
Submit
X
C
x 100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Choose the frequency of a peak that is most likely to correspond to an sp C-H stretch. A) 3450 cm-¹ 1 B) 3300 cm-¹ C) 3100 cm-¹ D) 2800 cm-¹ E) 2200 cm-¹arrow_forwardWhy is 7.28 R and 7.29 S? Because both of the 4 is on a line, and I thought that the configuration would only switch when the lowest priority was on the wedge.arrow_forward4arrow_forward
- 4. Draw an energy level diagram for bromine esc F tab 7 caps lock shift 19 NO O 80 2 3 A #3 1 control 888 19 S N S4 6 E 1 option 25 (5 R D X 94 78 T F Y G 1 3: * 00 2 H B 8: - 0⁰ ✓ N $10 O O K M P L < -arrow_forwardAnswer allarrow_forwardidentify the groups of homotopic protons and from these, the group with the lowest and the group with the highest shift values. Give an estimate for these shift values.arrow_forward
- Resources O Hint If a source radiates sound uniformly in all directions and you triple your distance from the sound source, what happens to the sound intensity at your new position? The sound intensity increases to three times its original value. The sound intensity does not change. The sound intensity drops to 1/3 of its original value. The sound intensity drops to 1/9 of its original value. The sound intensity drops to 1/27 of its original value. about us careers privacy policy terms of use contact us help 4:09 P 11/26/2arrow_forwardWhich of the following IR frequencies would be expected for ethanol? Select all that apply. ~1710 cm¹ (broad) ~2870 cm 1 O~2250 cm 1 0-3075 cm ¹ ~3410 cm1 (broad)arrow_forwardlabel major peaksarrow_forward
- How many 07 9 8 6 ¹HNMR 23) Reset Answer signals will observe for (2-bromopropyl)benzene?arrow_forwardConvert the following structural formulas into skeletal structures (or line formulas). Part 1 of 3 H H H-C-C-C H H Part 2 of 3 HICH x-U-x HHH H-C-C=C -C-H Click and drag to start drawing a structure. Click and drag to start drawing a || 9.arrow_forwardGiven this calibration curve for the Line Spectra lab, what is the correct equation of the trendline? Helium Calibration Curve 7.00E-07 y= 8.51E-10x + 6.42E-08 R = 1.00E+00 6.50E-07 6.00E-07 5.50E-07 5.00E-07 4.50E-07 4.00E-07 400 450 500 550 600 650 700 Scale (nm) R2 = 1.00E+00 7.00E-07 7.00E+0.00 y = 8.51E-10x + 6.42E-08 Wavelength (m)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY