
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
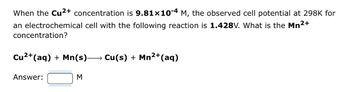
Transcribed Image Text:When the Cu²+ concentration is 9.81x10-4 M, the observed cell potential at 298K for
an electrochemical cell with the following reaction is 1.428V. What is the Mn²+
concentration?
Cu²+ (aq) + Mn(s)→→→→→→ Cu(s) + Mn²+ (aq)
2+
Answer:
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When the Cu2+ concentration is 1.00 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.799V. What is the Mg2+ concentration? Cu2+(aq) + Mg(s) Cu(s) + Mg2+(aq)arrow_forwardCalculate the cell potential for the following concentration cell at 25 °C. Ni(s) Ni(NO3)2(0.00522M)|Ni(NO3)2(3.07 M)Ni(s)arrow_forwardWhen the Cu2+ concentration is 1.00 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.799V. What is the Mg²+ concentration? Cu2*(aq) + Mg(s)- →Cu(s) + Mg²*(aq) Answer: Marrow_forward
- When the Hg2+ concentration is 1.11×10-4 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 1.916V. What is the Mn2+ concentration?Hg2+(aq) + Mn(s) Hg(l) + Mn2+(aq)arrow_forwardPayalarrow_forwardWhen the Pb2+ concentration is 5.46×10-4 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.147V. What is the Mg2+ concentration?Pb2+(aq) + Mg(s) Pb(s) + Mg2+(aq)arrow_forward
- Zn(s) │ Zn2+(aq, 0.10 M) ║ Zn2+(aq, 0.50 M) │ Zn(s) The concentration cell above was allowed to operate until the cell reaction reached equilibrium. What are the cell potential and the concentrations of zinc(II) in each half-cell for the cell now?arrow_forwardWhen the Hg2+ concentration is 1.63×104 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.401V. What is the A13+ concentration? 3Hg²+(aq) + 2Al(s)—>3Hg(1) + 2A13+(aq) Answer: Marrow_forwardWhen the Ag+ concentration is 4.19×10-4 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.257V. What is the Al3+ concentration? 3Ag+(aq) + Al(s)3Ag(s) + Al3+(aq)Answer: Marrow_forward
- When the Hg+ concentration is 8.47×104 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.422V. What is the Al3+ concentration? 3Hg*"(aq) + 2Al(s)- →3H9(1) + 2A1*(aq) Answer: 7.53x10^6arrow_forwardBased on the cell potentials in the previous question calculate equilibrium constant of the following reaction H2(g) + I2 (g) → 2HI(aq) Please enter your answer using E notation with 2 decimals. for example, 1234.456 is written as 1.23E3arrow_forwardUsing standard potentials given in the appendices, calculate the standard cell potentials for the following reaction: Cu(s) + 2Ag+ (aq) = Cu²+ + 2Ag(s) Report the answer in volts with three significant figures. 0.462 V Submit Previous Answers Correct Correct answer is shown. Your answer 0.46 V was either rounded differently or used a different number of significant figures than required for this part. Part B Determine the equilibrium constant for the above reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY