
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
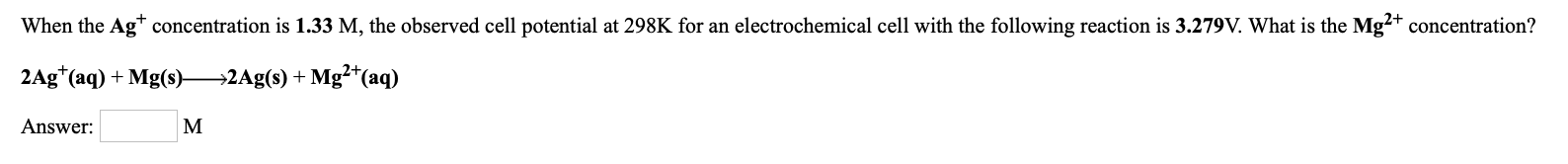
Transcribed Image Text:When the Ag* concentration is 1.33 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 3.279V. What is the Mg?+ concentration?
2Ag*(aq) + Mg(s)-
→2Ag(s) + Mg²*(aq)
Answer:
M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- When the Ag* concentration is 2.63×10-4 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 1.324V. What is the Cr³+ concentration? 3Ag*(aq) + Cr(s)- →3Ag(s) + Cr³+ (aq) Answer: Marrow_forwardWhen the Cu2+ concentration is 1.00 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 2.799V. What is the Mg²+ concentration? Cu2*(aq) + Mg(s)- →Cu(s) + Mg²*(aq) Answer: Marrow_forwardWhen the Hg2+ concentration is 2.22×10-4 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 1.486V. What is the Cr³+ concentration? 3Hg²*(aq) + 2Cr(s)3Hg(1) + 2C1³*(aq) Answer: Marrow_forward
- us the tabulated half cell potentials to calculate ▲G° for the following redox reaction 2Al(s) + 3Mg2+(aq) --> 2Al3+(aq) +3Mg(s)arrow_forwardWhen the Pb²+ concentration is 5.44×104 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 0.953V. What is the Mn²+ concentration? Pb²+ (aq) + Mn(s) Pb(s) + Mn²¹(aq) Answer: Marrow_forwardWhat is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the F2 pressure is 1.46 atm, the F" concentration is 6.08×10-3M, and the Pb2+ concentration is 5.78×10-4M ? F2(g) + Pb(s)- →2F*(aq) + Pb²+(aq) Answer: V The cell reaction as written above is spontaneous for the concentrations given:arrow_forward
- When the Hg2+ concentration is 1.33 M, the observed cell potential at 298K for an electrochemical cell with the following reaction is 3.335V. What is the Mg2+ concentration?Hg2+(aq) + Mg(s) =>Hg(l) + Mg2+(aq)Answer: Marrow_forwardSalt bridge A concentration cell similar to the one shown is composed of two Cr electrodes and solutions of different Cr3+ concentrations. The left compartment contains 0.222 M Cr3+ , and the right compartment contains 0.729 M Cr3+ . Calculate the cell potential for this reaction at 298 K. volts In this chromium concentration cell, the reaction would proceed spontaneouslyarrow_forwardWhat is the calculated value of the cell potential at 298K for an electrochemical cell with the following reaction, when the F2 pressure is 8.22×10-4 atm, the F concentration is 1.46M, and the Cr³+ concentration is 1.01M? 3F₂(g) + 2Cr(s)—6F¯(aq) + 2Cr³+ (aq) Answer: V The cell reaction as written above is spontaneous for the concentrations given:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY