
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
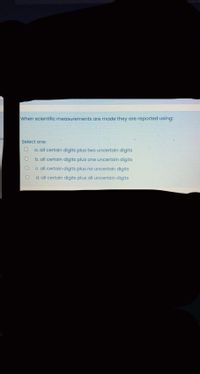
Transcribed Image Text:When scientific measurements are made they are reported using:
Select one:
a. all certain digits plus two uncertain digits
b. all certain digits plus one uncertain digits
C. all certain digits plus no uncertain digits
d. all certain digits plus all uncertain digits
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the following pairs of measurements, indicate which is the lesser (a or b). If two quantities are equal, write EQUAL. show your reasoning for points. A. a) 71% or b)0.77 B. a)2600nm or b)2.5μm C. a) 1.00 x 10^4 min or b) 1 week D. a) 2.000L or b)2000cm^3 E. a) 1.10Tb or b) 1000Gb F) a)10.0ft or b) 3.00marrow_forward1.Express the following numbers in scientific notation. a) 234000 b) 0.009190 c) 19500 d) 0.0124arrow_forwardTwo reasons to write a number in scientific notation are when it is either very big or very small. Another isarrow_forward
- 0 F3 4 $ Answer X f. 2.94 x 10-5 Answer+ 13. Write each of the following numbers in standard scientific notation. a. 1/1033 b. 1/105 c. 1/10-7 d. 1/0.0002 e. 1/3,093,000 f. 1/10 4 8.1/10⁹ h. 1/0.000015 14. Write each of the following numbers in standard scientific notation. a. 1/0.00032 RELTONGC MacBook Air F6 F7 O 000 000 F4 % 5 Pre H Re F5 < 6 & 7 * ► 11 8 F8 1 9 1 F9 O 1 0 FIarrow_forwardAn analog scale has marking for every 0.1 g. Which measurement is accurately reported for this scale? A. 20.g B. 20.4 g C. 20.36g D. 20.362arrow_forwardDetermine a single factor that can be used as a multiplier to 1.5 oz to gramsarrow_forward
- Below is data from a hypothetical experiment. The actual density of the substance is 2.37 g/mL. How would the quality of this data be characterized? Trial Density 2.21 g/ml 2.20 g/mL 1 2 2.22 g/mL 2.20 g/mL O a. Both precise and accurate O b. Neither precise nor accurate O C. Accurate O d. Precisearrow_forwardAn insulin syringe, a low volume syringe, commonly used for diabetic medication is intended for multiple use. Select one: a. False b. True Accuracy means Select one: a. All of these b. Closeness of a measured value to the real value C. A measure of how often an experimental value can be repeated d. The number of significant figures used in a measurement Basis for selecting the appropriate balance to use in weighing includes Select one: a. Precision and accuracy in weighing b. All these c. Weighing capaities d. Function that a balance performsarrow_forwardWhich of the following numbers conforms to the scientific notation conventions? A. 0.123 x 109 B. -1.23 x 1012 C. 1.23 x 102.1 D. 12.3 x 106arrow_forward
- Complete the following calculation. Report your answer with the correct number of significant figures. (7.339 x 2.3) + 27 Your Answer: Answerarrow_forwardWhich of the following has the smallest number of significant figures? Why? A. All of the other options have the same number of significant figures. B. 0.0050 C. 0.0500 D. 0.0005 E. 0.5000arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY