
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Solve all parts otherwise I will downvote
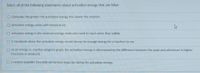
Transcribed Image Text:Select all of the following statements about activation energy that are false:
Generally, the greater the activation energy, the slower the reaction
activation energy varies with temperature
activation energy is the minimum energy molecules need to react when they collide
1 microjoule above the activation energy would always be enough energy for a reaction to run
on an energy vs. reaction progress graph, the activation energy is determined by the difference between the peak and whichever is higher
(reactants or products)
O a catalyst provides favorable elementary steps by raising the activation energy

Transcribed Image Text:When mixing which of the following pairs would you expect to have two hydrolysis reactions written?
O HF + HCIO4
O all of these
O HCI + CUOH
O HCIO2 + CuOH
O HCIO2 + KOH
O none of these
O HCI + Ba(OH)2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Match each pair of temperatures correctly. Drag statements on the right to match the left. 5°C 41°F 303 K 86°F -35°C 238 K Do you know the answer? I know It Think so Unsure No ideaarrow_forwardCa you please answer this in 10 minutes? pretty pleaaseee. Thank you ?arrow_forwardMust answer all questions eslse downvote A. MULTIPLE CHOICE. Choose the BEST answer. PLEASE HELP ME ANSWER EVERYTHING THANK YOU Q)What is used for heating small amounts of solids at a high temperature?a) Mortar and pestleb) Evaporating dishc) Crucible and coverd) Clay triangleQ)Which of the following is used in separation techniques?a-Rubber policemanb-Graduated cylinderc-Volumetric flaskd-Filter paperQ)Which of the following describes a centrifugate?a-Always clearb-Supernatant liquidc-Discarded via decantation onlyd-Solid particlesQThe inward force that pulls substances towards its center is called .a-Gravitational forceb-Centrifotal shiftc-Continental shiftd-Centrifugal forceQA "slippery floor" is considered a .a-hazardb-riskc-flash pointd-toxicantQ)Mrs. Lily Potter is 24 weeks pregnant. She was exposed to a chemical while making a potion. What is the type of the chemical she should be avoiding?a-neurotoxicantb-asphyxiantc-teratogend-carcinogenQ)Which of the following should be…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY