
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
need chemistry review help

Transcribed Image Text:4NO₂ (g) + O₂(g) = 2N₂0, (8)
Using the chemical equation you can write the pressure equilibrium constant expression equation:
Initial
Now comes the challenge. How are we going to find the equilibrium pressures to put into the right-hand side of this equation? One important clue is that you're
told the initial pressures. That suggests solving an equilibrium composition problem might be part of the solution. Let's go down that path a little and see what
happens.
To solve for the equilibrium composition, you'd begin by setting up an ICE table. So let's do that. We'll let stand for the rise in pressure of the NO:
Change
N₂0₂
NO₂
Equilibrium
Po
Jay₂0₂
pV=nRT
n= PV
RT
P
K =
P
4.0-2x
The problem, of course, is that we don't know X, and we can't solve for it without.
it. Are we stuck? Not necessarily. Let's think a moment about why knowing
would let us solve for X: it's because knowing that number gives us a relationship between the expressions in the "equilibrium" row of the ICE table. That is,
we're able to combine these expressions into an equation with just one unknown, the value of X. An equation with just one unknown can always be solved for
the value of the unknown using algebra.
NO₂
4.0
-2x
Is there any other relationship we can imagine between the entries in the ICE table? Some other way to get an equation involving X?
Maybe. The one other fact you're given is the mole fraction of N₂O, at equilibrium. That is, you're told the ratio of the moles of N₂O, to the total moles of
reactants and products. That's almost a relationship between the entries in the ICE table, except that it's in terms of moles. What we need is some way to
transform it into a relationship between pressures.
RT
PN₂0₂
PIOT
When the issue is put like that, hopefully the idea of the ideal gas law comes to mind, because you know the ideal gas law relates pressure and moles for gases.
Let's use the ideal gas law to figure out what relationship there is between the equilibrium pressure of N₂O, and the total pressure, given that we know the
relationship between the moles of N₂O, and total moles:
Px₂0₂
RT
PIOT
PIOT
P
9.1
1
-3x
9.1-x
P
Pos
0
Here's the ideal gas law.
Solving for tells us how moles can be expressed in terms of pressure.
Now we're getting somewhere. You're told ₂0₂² =0.11. That means you now know a relationship between the equilibrium pressure of N₂O, and the total
pressure. And that's all you need to solve for X, using the entries in the "equilibrium" row of the ICE table:
Here's the definition of the mole fraction of N₂O₂.
Here's what happens when we substitute in the ideal gas law expression for moles, top and bottom.
You can solve this equation for X using algebra:
x=0.11 (13.1-x)
1.165x-1.441=0
x= 1.237...
Everything cancels but the pressures!
(4.0-2x)-(9.1-x)+(x)
Clear fractions.
Multiply everything out.
Solve the linear equation.
The hard part is over. Now you just need to substitute this value for X back into the expressions in the "equilibrium" line of the ICE table, to get the equilibrium
pressures. These then go into the pressure equilibrium expression equation to give you
13.1-x
= 0.11
x²
(1.237)
(4.0-23)*(91-3) (4.0-2(1.237))*(9.1-(1.237))
= 0.03327...
21
Ar
H
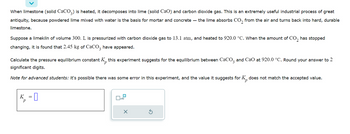
Transcribed Image Text:When limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great
antiquity, because powdered lime mixed with water is the basis for mortar and concrete - the lime absorbs CO₂ from the air and turns back into hard, durable
limestone.
Suppose a limekiln of volume 300. L is pressurized with carbon dioxide gas to 13.1 atm, and heated to 920.0 °C. When the amount of CO₂ has stopped
changing, it is found that 2.45 kg of CaCO3 have appeared.
Calculate the pressure equilibrium constant K this experiment suggests for the equilibrium between CaCO3 and CaO at 920.0 °C. Round your answer to 2
significant digits.
Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K does not match the accepted value.
K =
P
0
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- = Organic Molecules Assignment Draw the structure of iodobenzene. Check Click and drag to start drawing a structure. Terms of Use | Privacy Center Accessibility C™ X A ċ Save For Later tu Submit Assignmentarrow_forwardData Table 1. Compound Source Bees Gnnamon Coconut al Fish Garlic Lavender Lemon Mint Star Anise Stove Thyme Vanilla Extract IUPAC Namearrow_forwardQUESTION 11 When a substance undergoes a chemical change it is always true that ______________ heat is absorbed it changes state its chemical properties changes it changes colorarrow_forward
- Please correct answer and don't use hand ratingarrow_forwardKOVe Br 美 Select one: Previous page Exam-Onli *Week 11 5udentreadarrow_forwardCypress oil should be used _______ and should be stored in a _______ place. Choose one answer. a. diluted, warm b. diluted, cool c. undiluted, dark d. undiluted, lightarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY