
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
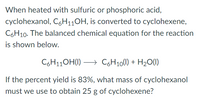
Transcribed Image Text:When heated with sulfuric or phosphoric acid,
cyclohexanol, C6H110H, is converted to cyclohexene,
C6H10. The balanced chemical equation for the reaction
is shown below.
C6H11OH(1)
→ C6H10(1) + H20(1)
If the percent yield is 83%, what mass of cyclohexanol
must we use to obtain 25 g of cyclohexene?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete combustion of 4.5892 a particular compound that contains only carbon, hydrogen and oxygen produces 10.4324 g of CO2 and 4.2705 g of H2O. The empirical formula of the compound is given by C H O (indicate "1" if there is only one atom of a given type in the empirical formula.) The molar mass of the compound is found to be 116.16 g/mol in a different experiment. What is the molecular formula of the compound? C H Oarrow_forwardThe reaction for producing glucose in plants, called photosynthesis, is 6CO2+6H2O→lightC6H12O6+6O2 If a plant produces 9.76 mol C6H12O6, how many moles of CO2 are needed?arrow_forwardWrite a balanced chemical equation based on the following description: butane gas reacts with oxygen gas to produce carbon dioxide gas and water vapor C4H10(g)+O2(g)_arrow_forward
- Hydrazine (N2H4) can be synthesized from ammonia (NH3) and hydrogen peroxide (H2O2) in the Peroxide process. The balanced chemical equation of this reaction is shown below: H2O2 (l) + 2NH3 (l) → 2H2O (l) + N2H4 (l) If 4 moles of ammonia were mixed with an excess amount of hydrogen peroxide, how many moles of hydrazine would be produced?arrow_forwardAqueous hydrochloric acid HCl reacts with solid sodium hydroxide NaOH to produce aqueous sodium chloride NaCl and liquid water H2O . If 2.19g of water is produced from the reaction of 24.4g of hydrochloric acid and 17.4g of sodium hydroxide, calculate the percent yield of water.arrow_forwardPredict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced and contains state symbols after every reactant and product. HCIO (aq) + H2O(l) → ローロ Х 5 2 00 Ararrow_forward
- 2 N₂O5(g) --> 4 NO₂(g) + O₂(g) True/False: The equation is already balanced as it is written.arrow_forwardAcrylonitrile is an important building block for synthetic fibers and plastics. Over 2 billion pounds of acrylonitrile are produced in the United States each year. The compound is synthesized from propene in the following reaction:2C3H6 + 2NH3 + 3O2 → 2C3H3N + 6H2OWhat mass of acrylonitrile can be prepared from 150.0 kg of propene, 600.0 kg of ammonia, and 100.0 kg of oxygen?arrow_forward2NO(g) + O2(g) → 2NO2(g) You are provided with 4.12 molmol of nitrogen monoxide gas. Using the balanced chemical equation completed in Part A, determine how many moles of oxygen gas are needed to completely react with the nitrogen monoxide gas and how many moles of nitrogen dioxide are formed as a result? Ammonia and oxygen react to form nitrogen monoxide and water. Construct your own balanced equation to determine the amount of NO and H2O that would form when 2.80 molmol NH3 and 4.64 mol O2 react. Express the amounts in moles to two decimal places separated by a comma.arrow_forward
- Balance the equation below. CO(g) + O2(g) → CO2(g) How many moles of CO(g) are required to react completely with 1 mole of O2(g)? Enter your response in moles (mol) to the nearest 0.1 mol.arrow_forwardFor the following reaction, 0.115 moles of aluminum oxide are mixed with 0.332 moles of sulfuric acid. aluminum oxide(s) + sulfuric acid(aq) → aluminum sulfate(aq) + water(l) What is the formula for the limiting reagent? What is the maximum amount of aluminum sulfate that can be produced? molesarrow_forwardAn intermediate step in the industrial production of nitric acid, HNO3, involves the reaction of ammonia, NH3, with oxygen gas, O2, to form nitrogen monoxide, NO, and water, H2O. 4 NH3 (g) + 5 O2(g) → 4 NO(g) + 6 H2O(g) Molar masses (g mol-1) NH3 = 17.03 O2 = 32.00 NO = 30.01 H2O = 18.02 (a) Assuming excess O2, how many grams of nitrogen monoxide, NO, (i.e., the theoretical yield of NO) can be formed by the reaction of 46.00 g of ammonia, NH3 b. Assuming excess NH3, what is the theoretical yield of NO if we start with 73.00 grams of O2? c.If we start with 8.0 moles of NH3 and 8.0 moles of O2, how many moles of NO will we produce? d. If we have 36.00 g of NH3 and 72.00 g of O2, what is the theoretical yield of NOarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Expert Answers to Latest Homework Questions
Q: You are interviewing three people for one sales job. On the basis of your experience, you believe…
Q: do fast all
Q: Kindly help me with accounting questions
Q: Not use ai please
Q: In Tyler LTD, direct materials are $12000, direct labor $15000 and manufacturing overhead is $9000.…
Q: Please provide this question solution general accounting
Q: Show work. don't give Ai generated solution
Q: You made a sale for $175,000. The customer paid in cash. Your gross margin is 48%. What is your cost…
Q: Answer this financial account question. In 5 min
Q: subject: general accounting
Q: Answer me this accounting reasoning Question
Q: Suppose the nation of Bittle produces only two goods, teapots and surfboards. If Bittle produces…
Q: Answer? ? Financial accounting
Q: Q2: The plate material of a pressure vessel is AISI 1050 QT 205 °C. The plate is rolled to a…
Q: Yuva Co. uses the percentage-of-receivables basis to record
bad debt expense and concludes that 3%…
Q: The capital stock is fixed at 40 units, the price of capital is
$15 per unit, and the price of labor…
Q: The capital stock is fixed at 40 units, the price of capital is $15 per unit, and the price of labor…
Q: B: Solid rotating shaft used in the boat with high speed shown in Figure. The amount of power…
Q: What is the earnings per share of this financial accounting question?
Q: Want ans accurately of this financial accounting problem
Q: The Perfect Lotus Co. has earnings of $2.00 per share. The benchmark PE for the company is 13. What…