
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
When comparing the electrode potential values for the following equations, which reaction more likely undergoes oxidation and which more likely undergoes reduction? Based upon the electrode potentials which electrode is the anode and which is the cathode?
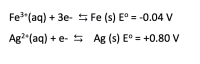
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which products are obtained in the electrolysis of molten NaI?arrow_forwardPlease explain and give the correct answers.arrow_forwardWrite a balanced chemical equation (including states of matter) and a build a cell diagram for an electrochemical cell that has a copper cathode immersed in a solution of Cu2+ ions, and a lead anode immersed in a solution of Pb2+ ions. what is the reaction: ?arrow_forward
- An electrochemical change occurs when a strip of nickel is immersed in an acidified solution that contains dichromate ions. The colour change that occurs as the reaction above proceeds is: Select one: Green to colourless Orange to green Colourless to green Green to orange Which of the following metals could have been used instead of nickel in order to maintain the original colour of the solution described above? Select one: O Gold Magnesium O Copper Cadmium Which of the following entities will react spontaneously with Cd(s) but not with Ni(s)? Select one: Sn²+ (aq) Cr²+ (aq) Coz+(aq) H₂Se(aq)arrow_forwardElement A has a standard reduction potential of +0.74V, whereas Element B has a standard reduction potential of -1.15V. Which of the following statements is correct with respect to building a Galvanic cell with these two elements? A. A will be the cathode, B will be the anode, and the cell potential will be positive. B. A will be the cathode, B will be the anode, and the cell potential will be negative. C. A will be the anode, B will be the cathode, and the cell potential will be positive. D. A will be the anode, B will be the cathode, and the cell potential will be negative. E. The anode, cathode, and cell potential must all be experimentally determined.arrow_forwardA galvanic (voltaic) cell contains a copper cathode immersed in a copper(I) chloride solution and a nickel anode immersed in a nickel(II) chloride solution. The two solutions are connected with a salt bridge. Write the balanced equation for the galvanic cell. Phases are optional. balanced equation: A current of 2.40 A is observed flowing through the cell for a period of 2.81 h. Calculate the amount of charge flowing through the circuit during this time. C Calculate the number of moles of electrons flowing through the circuit during this time. moles of clectrons: Calculate the mass change in grams of the copper and nickel electrodes. mass change of copper clectrodc: mass change of nickel electrode:arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY