
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
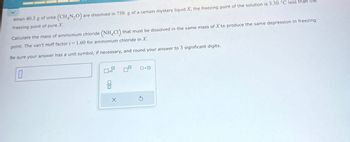
Transcribed Image Text:(CH,N,O). are dissolved in 750. g of a certain mystery liquid X, the freezing point of the solution is 3.30 °C less than the
When 40.3 g of urea
freezing point of pure X.
Calculate the mass of ammonium chloride (NH4Cl) that must be dissolved in the same mass of X to produce the same depression in freezing
point. The van't Hoff factor i=1.60 for ammonium chloride in X.
Be sure your answer has a unit symbol, if necessary, and round your answer to 3 significant digits.
0
00
X
ロ・ロ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Menthol is a crystalline substance with a peppermint taste and odor. When 1.10g of menthol is dissolved in 25.0 g of cyclohexane, the freezing point of the solution is lowered by 5.87 °C. The freezing point and Kf constant for cyclohexane can be found here. Calculate the molar mass of menthol.arrow_forwardThe freezing point of 57.08 g of a pure solvent is measured to be 50.83 ºC. When 2.86 g of an unknown solute (assume the van 't Hoff factor = 1.0000) is added to the solvent the freezing point is measured to be 48.78 ºC. Answer the following questions ( the freezing point depression constant of the pure solvent is 7.33 ºC·kg solvent/mol solute). Be sure to use the appropriate number of sig figs What is the molality of the solution? How many moles of solute are present? What is the molecular weight of the solute?arrow_forwardA solution of 4.55 % phenol (C6H6O) solution by mass in carbon tetrachloride, CCl4. The density of the solution is 1.602g/mL. What are the molality, mole fraction, and molarity of phenol in the solution? a. Fill in the summary table as you determine different parts of the problem – the first few columns are labeled – it is up to you to decide what is important information: Molar mass mole Solute Solvent Solution b. Molality: c. Mole Fraction: d. Molarity:arrow_forward
- A solution consisting of 0.240 mol of methylbenzene, C6H5CH3, in 252 g of nitrobenzene, C6H5NO2, freezes at –0.7°C. Pure nitrobenzene freezes at 6.0°C. What is the freezing-point depression constant of nitrobenzene?arrow_forward5 L山 When 1.06 g of a certain molecular compound X are dissolved in 85.0 g of dibenzyl ether ((C,H,CH,),0), the freezing point of the solution is measured to be 0.9°C. Calculate the molar mass of X. If you need any additional information on dibenzyl ether, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to 1 significant digit. 自 OL Check Save For Later Submit Assignment O2022 McGraw Hill LLC. AlI Rights Reserved. Terms of Use Privacy Center Accessibility EPIC 中Xe MacBook Pro F2 F3 F4 F7 F8 F10 6-5 #3 3. 2 $ 一 8 dele R. A C. W alt alt option 10 command option 1Q15arrow_forwardSuppose that you add 21.1 g of an unknown molecular compound to 0.250 kg of benzene, which has a Kf of 5.12 °C/m. With the added solute, you find that there is a freezing point depression of 2.86 °C compared to pure benzene. What is the molar mass (in g/mol) of the unknown compound?arrow_forward
- When 2.85 g of a certain molecular compound X are dissolved in 85. g of dibenzyl ether ((CH₂CH₂)₂O), the freezing point of the solution is measured to be 2 -1.2 °C. Calculate the molar mass of X. If you need any additional information on dibenzyl ether, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. X x10 Śarrow_forwardWhen 4.18 g of a certain molecular compound X are dissolved in 45.0 g of benzene (C6H6), the freezing point of the solution is measured to be 4.9 °C. Calculate the molar mass of X. If you need any additional information on benzene, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. □□ 品 5arrow_forward1.613 g sample of an unknown nonionizing molecular compound is added to 250.0 g of benzene(C6H6, MW = 78.11 g/mol). The normal freezing point of the solution is 0.17 C lower than the normalfreezing point of pure benzene. Based on this information, find the molecular weight of the unknowncompound. benzene = Kf = 5.12 kg x Degrees C/mol.arrow_forward
- When 14.4 g14.4 g of an organic compound known to be 55.81% C55.81% C, 7.0% H7.0% H, and 37.17% O37.17% O by mass is dissolved in 825.8 g825.8 g of benzene, the freezing point is 4.80 ∘C4.80 ∘C. The normal freezing point of benzene is 5.49 ∘C5.49 ∘C. What is the molecular formula for the organic compound? Assume that the organic compound is a molecular solid and does not ionize in water. ?fKf values for various solvents are given in the colligative constants table. molecular formula: CHOarrow_forwardAt a certain temperature the vapor pressure of pure thiophene (C,H,S) is measured to be 0.37 atm. Suppose a solution is prepared by mixing 106. g of thiophene and 93.4 g of acetyl bromide (CH,COB1). Calculate the partial pressure of thiophene vapor above this solution. Round your answer to 2 significant digits. Note for advanced students: you may assume the solution is ideal. atmarrow_forwardWhen 1.38 g of a certain molecular compound X are dissolved in 90.0 g of cyclohexane (C,H,,), the freezing point of the solution is measured to be 6.3 °C. Calculate the molar mass of X. If you need any additional information on cyclohexane, use only what you find in the ALEKS Data resource. Also, be sure your answer has a unit symbol, and is rounded to the correct number of significant digits. x10arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY