
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
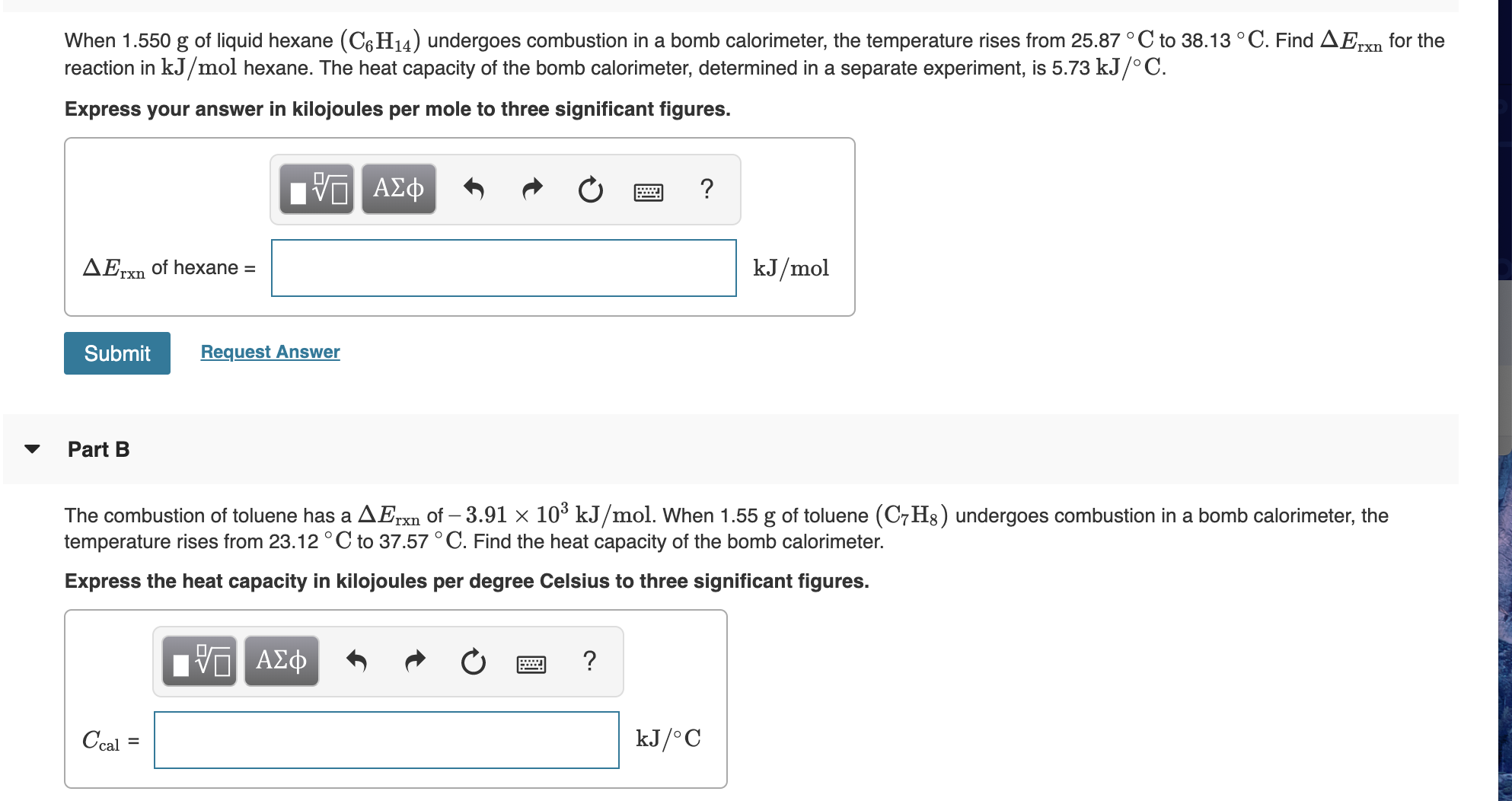
Transcribed Image Text:When 1.550 g of liquid hexane (C6H14) undergoes combustion in a bomb calorimeter, the temperature rises from 25.87 °C to 38.13 °C. Find AErxn for the
reaction in kJ/mol hexane. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.73 kJ/° C.
Express your answer in kilojoules per mole to three significant figures.
nνα ΑΣφ
kJ/mol
AErxn of hexane =
Request Answer
Submit
Part B
The combustion of toluene has a AErxn of – 3.91 × 10³ kJ/mol. When 1.55 g of toluene (C7H8) undergoes combustion in a bomb calorimeter, the
temperature rises from 23.12 ° C to 37.57 °C. Find the heat capacity of the bomb calorimeter.
гxn
Express the heat capacity in kilojoules per degree Celsius to three significant figures.
nν ΑΣφ
kJ/°C
Ccal =
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- A 55.1g sample of polystyrene, which has a specific heat capacity of 1.880·J·g−1°C−1, is put into a calorimeter (see sketch at right) that contains 200.0g of water. The temperature of the water starts off at 25.0°C. When the temperature of the water stops changing it's 31.9°C. The pressure remains constant at 1atm . Calculate the initial temperature of the polystyrene sample. Be sure your answer is rounded to the correct number of significant digits.arrow_forwardAcetylene, C 2 H 2 , is used in welding torches. It releases a lot of energy when burned in oxygen. The combustion of one gram of acetylene releases 48.2 kJ. A 0.750-g sample of acetylene is burned in a bomb calorimeter ( heat capacity = 1.117 kJ / °C ) that contains 800.0 g of water. The final temperature of the bomb and water after combusion is 35.2°C. What is the initial temperature of the bomb and water?arrow_forwardWhen 1.836 grams of sucrose (Molar mass 342.3 g/mol) is burned in a bomb calorimeter, the temperature of the calorimeter increases from 22.41°C to 26.63°C. If the heat capacity of the calorimeter is 4.900 kJ/°C, what is the heat of combustion of sucrose?arrow_forward
- A 2.30 g sample of ethanol, CH3CH2OH, is combusted in a bomb calorimeter. The temperature of the calorimeter increases by 4.89 K. If the heat capacity of the bomb is 663 J/K and it contains 2.57 kg of water, what is the enthalpy change per mole of ethanol combusted? The specific heat capacity of water is 4.18 J/g×K and the molar mass of ethanol is 46.07 g/mol.arrow_forwardSuppose you burned 0.300 g of C(s) in an excess of O₂ (g) in a constant-volume calorimeter to give CO₂ (g). C(s) + O₂(g) → CO₂ (g) The temperature of the calorimeter, which contained 776 g of water, increased from 24.90 °C to 27.28 °C. The heat capacity of the bomb is 896 J/K. Calculate AU per mole of carbon. (The specific heat capacity of liquid water is 4.184 J/g. K.) AU = = kJ/mol Carrow_forwardWhen 1.550 g of liquid hexane (C6H14) undergoes combustion in a bomb calorimeter, the temperature rises from 25.87 °C to 38.13 °C. Find AErxn for the reaction in kJ/mol hexane. The heat capacity of the bomb calorimeter, determined in a separate experiment, is 5.73 kJ/°C. Express your answer in kilojoules per mole to three significant figures. AErxn of hexane = Submit VE ΑΣΦ Request Answer ? kJ/molarrow_forward
- When a 4.31 g sample of liquid octane (C8H18) is burned in a bomb calorimeter, the temperature of the calorimeter rises by 27.3 °C. The heat capacity of the calorimeter, measured in a separate experiment, is 6.2 kJ/•C. The calorimeter also contains 3.00 kg of water, specific heat capacity of 4.18 J/g°C. Determine the heat of combustion of octane in units of kJ/mol octane. Enter your answer numerically and in terms of kJ/mol.arrow_forwardWhen 9.42 g of methane (CH4) is burned in a bomb calorimeter (heat capacity = 2.677 × 103 J/°C), the temperature rises from 24.00 to 27.08°C. Calculate the enthalpy change for the following reaction. (C= 12.00 amu, H = 1.00 amu) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) Group of answer choices a. 8.425 kJ b. –14.01 kJ c. –8.425 kJ d. 8245 J e. 14.00 kJarrow_forwardA new material was created in the lab. In order to determine the molar heat capacity, Cm, of the material 23.198 g of the substance was heated from 21.1ºC to 41.19ºC, which required 118.634 J of heat. The molar mass of the substance was determined to be 50.51 g/mol. Report the molar heat capacity in units of J/(mol · ºC) to 4 sig figs, but do NOT include units in your answer.arrow_forward
- The combustion of toluene has a AErxn of -3.91 x 10³ kJ/mol. When 1.30 g of toluene (C7H8) undergoes combustion in a bomb calorimeter, the temperature rises from 22.80 °C to 36.11 °C. Find the heat capacity of the bomb calorimeter. Express your answer using three significant figures. Ccal = 17| ΑΣΦ ? kJ/°Carrow_forwardAn 8.0 g sample margarine was placed in a bomb calorimeter. It was then completely combusted leading to a temperature change of 36.18 °C. The heat capacity of the calorimeter was previously determined to be 7.25 kJ/kg°C. What was the quantity of heat released to the environment by the sample of margarine?arrow_forwardPart A A silver block, initially at 55.8 °C, is submerged into 100.0 g of water at 24.1°C in an insulated container. The final temperature of the mixture upon reaching thermal equilibrium is 26.3 °C. The specific heat capacities for water and silver are = 4.18 J/(g ·°C) and 0.235 J/(g·°C). What is the mass of the silver block? Express your answer to two significant figures and include the appropriate units. Cs, water • View Available Hint(s) Cs, silver = HA ? Value Units m =arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY