
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
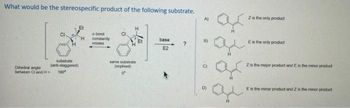
Transcribed Image Text:What would be the stereospecific product of the following substrate.
substrate
(ant-staggered)
180"
Dihedral angle
between Cl and H=
abond
constantly
rotates
same substrate
(explised)
base
E2
?
A)
B)
6
ar
ar
Z is the only product
Es the only product
Z is the major product and E is the minor product
Es the minor product and 2 is the manor product
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the substrates below can not undergo an E2? CH;CI Br OTs А C O A, B, and E O B, C, and D O A, B, C, D, and E B, D, and E O A, B, and C O A, B, and Darrow_forward10. a) In the box at the left, indicate whether the reaction takes place through an SN2, SN1, E2, or E1 mechanism. b) Draw the mechanism and predict the product, including stereochemistry. OH H3O* heat CI NaCN DMF KOt-Bu Br HOH HCIarrow_forwardDraw the intermediate and major product structurearrow_forward
- Q18. Predict the major product from the reaction shown below and draw the curved arrow mechanism for the electrophilic chlorination. OMe Cl2, FeCl3 ထု OMearrow_forwardDetermine if the conditions in each reaction below will favor an SN2 or an E2 mechanism as the major pathway. Then draw the major product that results.arrow_forward(There was an extra carbon, so those extras were whited out)arrow_forward
- 4. Predict the products for the following enolate reactions. a) LDA b) MeBr a) 0.95 equiv LDA RT b) MeBr a) 1.1 equiv LDA -78 C b) c) H3O+ Harrow_forwardPlease predict the outcome of each E2 elimination reaction: Hint: Assume it can only occur if the beta-hydrogen atom is anti-periplanar to the leaving group. Type you answer as a letter: A, B, or F. Problem 1: Answer to Problem 1: D A DH H H D CH3 "CI CH3 CH3 CH3 anti- ***** E2 elimination H H B E ? CH3 CH3 CH3 C F H CH3 CH3 CH3arrow_forward1) Which substrate would react most rapidly in an SN2 reaction? O CH3CH2CH2CH=CHBr OCH2=CHCH2CH2CH2Br CH3CH2CH=CHCH2Br BrCH2CH2CH=CHCH3 OCH3CHBRCH=CHCH3arrow_forward
- 2. Complete one of the SN2 reactions below that occurs with an enolate nucleophile. 1) LDA, -78 °C a) 2) Br 1) LDA, -78 °C CB Chistry Sto b) Chemistry Steps 2) c) 1) LDA, -78 °C 2) Br 3) Br₂, H+ Ⓒ3) Chemistry Ste 4) Pyridine CS Chemistry Steps 1) LDA, -78 °C 1) NaH ? e) ? 2) Chemistry Steps 2) Br Chemistry Steps Chemistry Steps Chemistry Steps Chemistry Stepsarrow_forwardDraw the full mechanism for the following transformationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY