
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What would be the net ionic equation?
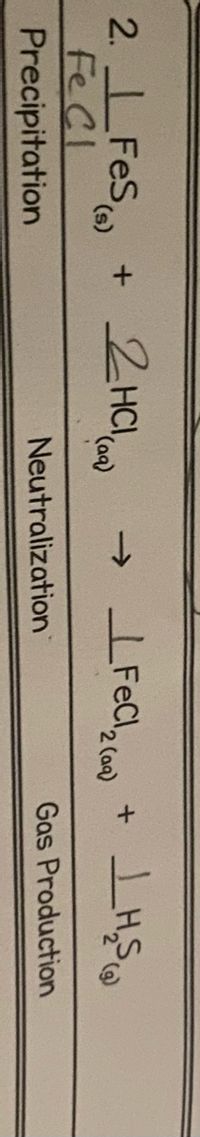
Transcribed Image Text:2. FeS
FeCl
Precipitation
2HCI,
O
(a)
(s)
Neutralization
Gas Production
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- According to the Resource Conservation and Recovery Act (RCRA), waste material is classified as toxic and must be handled as hazardous if the lead concentration exceeds 5 mg/L. By adding chloride ion, the lead ion will precipitate as PbCl2, which can be separated from the liquid portion. Once the lead has been removed, the rest of the waste can be sent to a conventional waste treatment facility. How many grams of sodium chloride must be added to 500 L of a waste solution to reduce the concentration of the Pb2+ ion from 10 to 5 mg/L?arrow_forwardThe equation for a reaction by which a solution of sodium carbonate may be standardized is 2HC7H5O2+Na2CO32NaC7H5O2+H2O+CO2. A student determines that 5.038g of HC7H5O2 uses 51.89mL of sodium carbonate solution in the titration. Find the molarity of the sodium carbonate.arrow_forwardSilver ions can be found in some of the city water piped into homes. The average concentration of silver ions in city water is 0.028 ppm. (a) How many milligrams of silver ions would you ingest daily if you drank eight glasses (eight oz/glass) of city water daily? (b) How many liters of city water are required to recover 1.00 g of silver chemically?arrow_forward
- Potassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory as a primary standard. It has the formula KHC8H4O4. This is often written in the short-hand notation as KHP. If 25.0mL of a potassium hydroxide solution are needed to neutralize 2.26g of KHP, what is the molarity of the potassium hydroxide solution? Potassium hydrogen phthalate sometimes called potassium biphthalate, as shown on this bottle is an acid that is convenient to store and use because it is a solid.arrow_forwardWhen jump-starting a car with a dead battery, the ground jumper should be attached to a remote part of the engine block. Why?arrow_forward68. Aluminum ion may be precipitated from aqueous solution by addition of hydroxide ion, forming Al(OH)3. A large excess of hydroxide ion must not be added, however, because the precipitate of Al(OH)3 will redissolve as a soluble compound containing aluminum ions and hydroxide ions begins to form. How many grains of solid NaOH should be added to 10.0 mL of 0.250 M A1Cl3 to just precipitate all the aluminum?arrow_forward
- When 85.0 mL of 0.250 M Ba(OH)2 solution is added to 85.00 mL of 0.250 M Al (NO3)3 solution, a white gelatinous precipitate of Al(OH)3; is formed. Assuming 100% yield, (a) what mass (in grams) of Al(OH)3 is formed? (b) what is the molarity of each of the ions Ba2+, OH-, Al3+, NO3- in the resulting solution?arrow_forwardTwenty-five milliliters of a solution (d=1.107g/mL)containing 15.25% by mass of sulfuric acid is added to 50.0 mL of 2.45 M barium chloride. (a) What is the expected precipitate? (b) How many grams of precipitate are obtained? (c) What is the chloride concentration after precipitation is complete?arrow_forward(Last Day Promotio... Kronos Worktorce... Maame Acheampo... MIDTERM M [Review Topics] [References] Use the References to access important values if needed for this question. How many grams of Ag,CO3 will precipitate when excess Na,CO3 solution is added to 77.0 mL of 0.690 M AGNO, solution? 2AGNO3(aq) + Na,CO3(aq) Ag,CO3(s) + 2NAN03(aq) + 2NaNO3(aq) Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
- A 0.450 M solution of KCIneeds to be prepared throughdilution. A 2.00 M stocksolution will be added to a0.250 L volumetric flask andthen water will be added to the0.250 L mark.Determine the volume (in mL)of the 2.00 M stock solutionof KCl needed to produce thissolution.arrow_forwardcoulbonug9 oinihe olni bouogmoOB QU llaa of word Solubility Rules: Water Soluble Compounds EXSube sCHcood) or etosiq 1. Salts of ammonium (NH4*) and Group IA are always soluble. 2. Salts containing nitrate (N03-), acetate (C2H3O2-), or chlorate (CIO3-) ions are always soluble. enoiteo 1ol ooJ 3. Salts of the halides are soluble with the following exception: a. Compound containing Ag*, Hg2*, Pb²* prirdolaw Jon er Es i olnol the exception of: 4. Salts containing the sulfate ion (SO42-) are soluble elgmsx3 a. Compounds containing Ag*, Hg2*, Pb²+, Ca2*, Sr2*, or Ba2+ Water Insoluble Compounds Juloe ent! 1. Salts of the carbonate (CO32-), phosphate (PO43-), chromate (CrO42-), or sulfides (S2-) ions are insoluble with the exception of compound containing group 1A or ammonium cations. 2. Salts containing the hydroxide ion (OH-) are insoluble with the exception of compounds containing group 1A, ammonium cations, or Ba2+. biup Inot luo tneioliteoo belon Soluble means that it dissolves in water…arrow_forwardPlease answer all partsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
