
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What would be the net ionic equation?
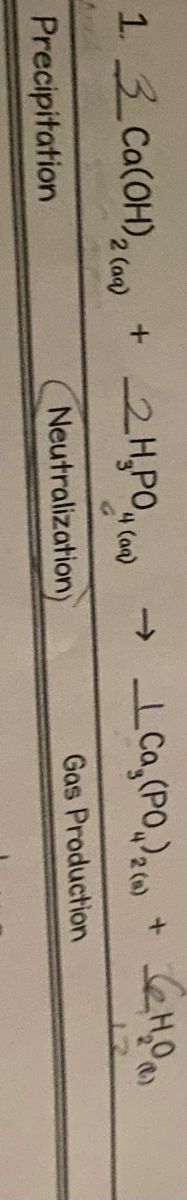
Transcribed Image Text:13 Ca(OH), ()
2H,PO,
2 (aq)
4 (aqa)
->
2 (
Precipitation
Neutralization)
Gas Production
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What mass of Cs2SO4 must be dissolved in enough water to give 447. mL of solution having a final cesium ion concentration of 7.62 M?The molar mass of Cs2SO4 is 361.873 g/mol.arrow_forwardComplete the balanced molecular chemical equation for the reaction below. If no reaction occurs, write NR after the reaction arrow. LiCl(aq)+K₃PO₄(aq)→arrow_forward1. Write the correct skeleton chemical equation for the following reaction: solid aluminum bromide and chlorine gas react to form solid aluminum chloride and liquid bromine 2. Write the skeleton reaction equation for the following word reaction equation: Aqueous copper(II) sulfate reacts with sodium hydroxide dissolved in water to yield aqueous sodium sulfate and solid copper(II) hydroxidearrow_forward
- How might you use a precipitation reaction to prepare a sample of Cu(CO3)? Write the net ionic equation.arrow_forwardWrite an equation for the precipitation reaction that occurs (if any) when solutions of sodium hydroxide and barium nitrate are mixed.arrow_forwardWrite an equation for the precipitation reaction that occurs (if any) when solutions of sodium oxalate and barium nitrate are mixed.arrow_forward
- In the laboratory, a student dilutes 25.3 mL of a 8.46 M hydrobromic acid solution to a total volume of 100.0 mL. What is the concentration of the diluted solution?arrow_forwardDesigning Chemical Reactions Lab Activity Given the list of chemicals below, write a balanced chemical equation that satisfies the reaction described. Write a description of what signs of a chemical change you would expect to see if you were able to do the reaction and write the total and net ionic equations for each reaction. Metals Bases Compound Solutions. copper (II) sulfate Ferric nitrate Acids sulfuricic acid Magnesium sodium hydroxide Indicators phenolphthalein litmus paper Zinc Calcium potassium iodide potassium nitrate Copper Iron sodium carbonate magnesium nitrate iodine solution. Single Displacement Reactions: ● Reaction #1 - Pick one reaction that works using the metal activity series: Indication of a chemical change: Total lonic Equation: Net Ionic Equation: Reaction #2 - Pick one reaction that works with water: Indication of a chemical change: Total lonic Equation: Net Ionic Equation: Reaction #3 - Pick one reaction that does not work using the halogen series: Indication…arrow_forwardwrite a net ionic equation for the reaction that occurs when manganese (II) suflide and excess hydrochloric acid (aq) are combinedarrow_forward
- 8)Does a reaction occur when aqueous solutions of potassium hydroxide and sodium nitrate are combined? yes or no If a reaction does occur, write the net ionic equation.arrow_forwardIn the laboratory, a student dilutes 29.1 mL of a 9.35 M hydrobromic acid solution to a total volume of 250.0 mL. What is the concentration of the diluted solution?arrow_forwardPredict the reactants of this chemical reaction. That is, fill in the left side of the chemical equation. Be sure the equation you submit is balanced. (You can edit both sides of the equation to balance it, if you need to.) Note: you are writing the molecular, and not the net ionic equation. [] + KCIO (aq) + H_o(1) → + H2O ローロarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY