
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
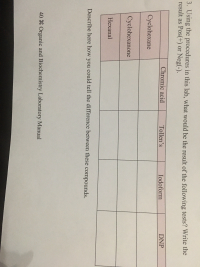
What would be a result of the tests
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Why does the water go up to the bottle? I need claim, evidence and reasoningarrow_forwardHow did you get 0.32?Also where did you get 500 for Vmax? In the question it says 50arrow_forwardWhat information should the RESULTS AND DISCUSSION section of a chemistry lab report contain? Select all that apply explanation of the importance of the data Cal figure(s) showing trends in the data of O an overview of how the experiment was conducted table(s) of data acquired in the experiment O an explanation of any errors that may have affected the data aarrow_forward
- Three trials for this experiment had been conducted and the following are the masses of limiting reactant: 0.285 grams, 0.299 grams, and 0.292 grams. What is the standard deviation for the masses of limiting reactant for the three trials? please show work on how to do.arrow_forwardCalculating BMI with English Units Khloe is 5 feet, 9 inches (5’9”) and 170 lbs. Calculate her BMI. (Round to the nearest 0.1.) what is her weight statusarrow_forwardWrite a paragraph discussing the accuracy of these measuring devices and theiruses? Look at the attached it is the percent error for 3 measuring devices then answer the question above (100ml Beaker 25 ml graduated cylinder and p1000(1000uL) Micropipette)arrow_forward
- After analyzing soil samples, we found 70 mg arsenic in 120 kg soil. Concentration of arsenic in the soil = ________ µg/kgarrow_forwardUsing the image what is the volume of the chalk? In mLarrow_forwardFor which pieces of lab equipment will mass measurements be recorded using the analytical balance? Select one: Watchglass Weighing dish Rubber stoppers Corks and vial with caparrow_forward
- Question 2 Using a calibration curve, the measured response obtained from an unknown sample of an environmental toxin is compared against, O The measured response of another unknown sample of the same environmental toxin. O The measured responses of samples of the same toxin at unknown concentrations. O The measured responses of standards of the same toxin at known concentrations. O The measured responses of different environmental toxins at known concentrations.arrow_forwardA student obtains three liquids (A, B & C) in three seperate test tubes. The densities for liquids A, B and C are found to be 2.3 g/ml, 0.95 g/ml, and 0.67 g/ml respectively. Liquid A is poured into Liquid B. Two layers appear. What liquid is likely to be in the top layer?arrow_forwardA jar test indicates that the best dry Alum dose is 8 mg/l. If the flow is about 2.6 MGD, what is the desire Alum feed rate in pounds per day? Group of answer choices 64 lbs/day 123 lbs/day 173 lbs/day 350 lbs/dayarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY