
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
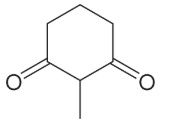
What will the enolate for this be using LDA, THF, and cold temperatures? What will it be using NaOEt at rt?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 6 images

Knowledge Booster
Similar questions
- Draw the electrophile formed by the following reagents/conditions below.arrow_forwardis this of the key imediate?arrow_forwarda. Draw the structure of the tetrahedral intermediate INITIALLY-FORMED in the reaction shown. H20 NH H2SO4 You do not have to consider stereochemistry. Do not include counter-ions, e.g., Na™, I¯, in your answer. In cases where there is more than one answer, just draw one. b. Draw the structures of the organic products of the acyl transfer reaction. You do not have to consider stereochemistry. Draw the neutral form of the products; no charges. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu.arrow_forward
- Q) can you show the step by step mechanism of these stating the neuclophiles and electrophiles and discussing the stereoselectivity? Why is that one has a higher yield then the other? Thank youarrow_forwardIs true about halohydrins, example bromohydrin the intermediate in a. the reaction mechanism is a cyclic halonium (bromonium) ion all b. alternatives are correct c.the product contains a halogen and an OH on adjacent carbons d. the nucleophile (H2O) attacks the most substituted carbonarrow_forwardQuestion 25 What reagents are needed to carry out the conversion shown? EtO Et Et Он O 1. HOCH2CH2CH3/H2SO4; 2. LIAIH4; 3. H3O* O 1. HOCH2CH2OH/H2SO4; 2. AICI3; 3. H30* О 1. НОСН2СН2ОН; 2. AICI3; 3. Нзо" O 1. HOCH2CH2CH2CH2OH; 2. LIAIH4; 3. H2SO4 O 1. HOCH2CH2OH/H2SO4; 2. LIAIH4; 3. H3O*arrow_forward
- What will be in the HNMR of N,N-dimethylformamide (DMF), and ethyl acetate? Be clear and neat, please!arrow_forwardplease draw a curved arrow mechanism for the folloeing reaction. please include all intermediate steps H -NR -BuMe₂SICI, Et3N, DMF (50% overall yield from L-aspartic acid (12)) -NHarrow_forwardDraw the organic product of the nucleophilic substitution reaction. Include all hydrogens atoms. CH3CH₂I + CH3CH₂O¯ → Select Draw Templates More //// C 0 H Erase Q2Qarrow_forward
- In Part 1 add the curved arrows to the nucleophilic acyl substitution reaction mechanism. In Part 2, answer the multiple-choice question about the reaction in Part 1. Part 1 See Periodic Table O See Hint :ö: :Br: Add the missing curved arrow notation. 20 F CI Br I Part 2 Which statement is most correct regarding the equilibrium for the above reaction? Choose one: O The equilibrium favors the right (i.e., Kea > 1) because acetate is a better leaving group than bromide. O The equilibrium favors the right (i.e., Keg > 1) because bromide is a better leaving group than acetate. O The equilibrium favors the left (i.e. Keg « 1) because acetate is a better leaving group than bromide. O The equilibrium favors the left (i.e., Keg« 1) because bromide is a better leaving group than acetate.arrow_forwardNeed help on this question. is it that they are in the same category because of the strong OH activator ?arrow_forwardIn the anhydride compounds, the Sym stretching of the carbonyl groups Higher than Asym stretching .A O Smaller than Asym stretching .B O No relation between them.c O Equal.D Oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole