
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
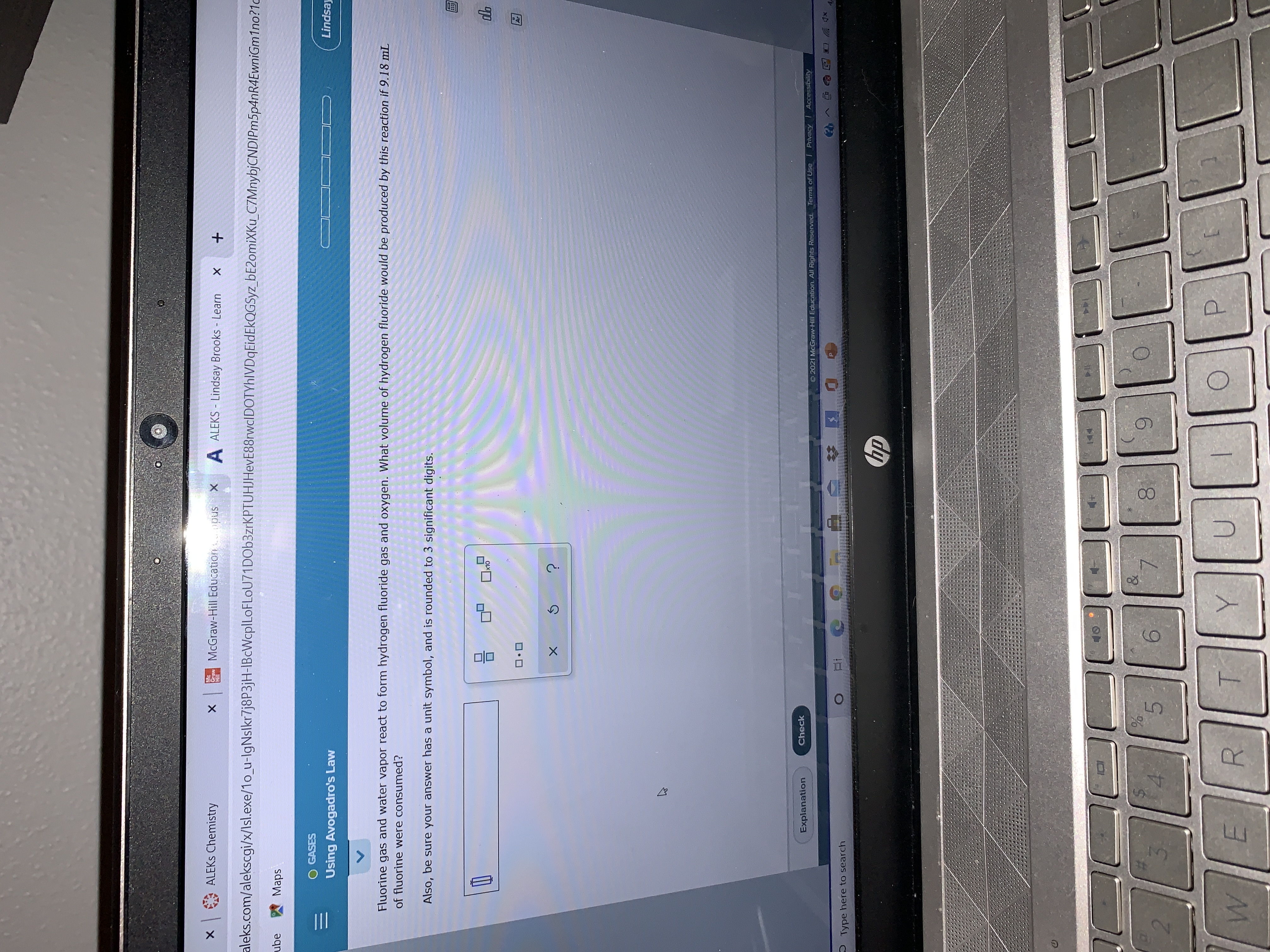
Transcribed Image Text:What volume of hydrogen fluoride would be produced
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate grams of NH3 formed when 34.0 L of hydrogen gas reacts with excess nitrogen gas. Write and balance the reaction. Show calculations with appropriate significant figures and unit analysis.arrow_forwardIf 26.8 grams of water react to form 3.0 grams of hydrogen, how many grams of oxygen must simultaneously be formed?arrow_forwarduse 17.89 mL (experimental volume) and 0.00074044 mol H2 (theoretical moles of hydrogen gas) to calculate the value of R. Include correct unitsarrow_forward
- How many grams of hydrogen reacted with nitrogen to produce 60.3 grams of ammonia?arrow_forwardIn an industrial process, hydrogen chloride, HCl, is prepared by burning hydrogen gas, H2, in anatmosphere of chlorine, Cl2. Write the chemical equation for the reaction. Below the equation, give themolecular molar and mass interpretations.arrow_forwardWhen 4.69 g of ssulfur combines with flourine, 15.81 g of gas is produced. a. what is the empirical formula of the gas? b.assuming that the empirical and molecular formulas are the same, what is its name?arrow_forward
- Please don't provide handwriting solutionarrow_forward27.0 f of copper is converted to copper (II) oxide by heating in oxygene. what mass of oxygen is required to convert the 57.0 of copper into copper II oxide?arrow_forward4.62 g sample of methane (CH4) is reacted with 15.1 g of oxygen. What is the maximum amount of water (theoretical yield) which could be formed?arrow_forward
- How many grams is equivalent to 1.204x1024 molecules of H2O?arrow_forwardSolid lithium hydroxide is used to remove exhaled carbon dioxide from space vehicles by forming lithium carbonate and liquid water. What mass of carbon dioxide can be absorbed by 86.20 grams of lithium hydroxide?arrow_forward11. How much iron, in kg, would need to be used in excess oxygen to produce 1,679 g of rust (iron(III) oxide)? use dimensional analysis to complete this questionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY