
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please give handwritten answer. ASAP
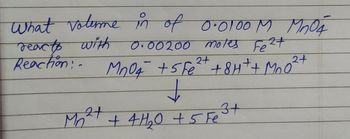
Transcribed Image Text:What volume in of 0.0100 M MnO4
reacts with
0.00200 moles Fe²+
Reaction:- MnO4 + 5 Fe²+ +8H+ + MnO²
2+
2+
↓
3+
2+
Mn²+ + 4H₂0 + 5 Fe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 22-30. (a) The mean free path is the average distance a molecule travels before colliding with another molecule. The mean free path, A, is given by A = kT/(V20P), where k is Boltzmann’s constant, T is temperature (K), P is pressure (Pa), and o is the collision cross section. For a molecule with a diameter d, the col- lision cross section is nd. The collision cross section is the area swept out by the molecule within which it will strike any other molecule it encounters. The magnetic sector mass spectrometer is maintained at a pressure of ~103 Pa so that ions do not collide with (and deflect) one another as they travel through the mass analyzer. What is the mean free path of a molecule with a diam- eter of 1 nm at 300 K in the mass analyzer?arrow_forwardC7 Q7:arrow_forwardFigure 7 of Conrad et al. (1999) shows the variation in C-isotopic composition of CO2 as a function of the concentration of methane measured in the soil and aquifers of a site contaminated in the residues of aviation fuels. Explain briefly the aim of these particular measurements made to the environmental study. Also explain briefly why there is a difference in isotopic composition of CO2 obtained via aerobic degradation and methanogenic degradation of AVGAS (fuel residues as contaminants) and the linear “mixing relationship” noted in Figure 7. How does the degradation process influence the CO2 isotopic signature ? I don't understand why the CO2 from aerobic degradation is lighter than the one from methnogenic degradation ?arrow_forward
- describes the structure formed from the FTIR results above and relates it to the lignin and cellulose content contained in the sample after the delignification processarrow_forward3arrow_forwardDr. Alghe has just performed a restriction digest on DNA she extracted from her samples. She must now run her DNA out on a gel to separate the fragments based on size. To do this she must prepare a 1.2% agarose gel, where the percentage is determined as mass/vol. How many grams of agarose must she add to 200 mL of buffer in order to arrive at the correct percentage?arrow_forward
- ucose-cocp-2000+100g ge Find Cyoy the value of the [HgCl2] (M) [C2042-1 (M) | Rate (Mis) 0.10 0.10 1.3.10-7 0.10 0.20 5.2.10-7 0.20 0.20 1.0-10-6 A) 1.4 x 10-8 1/M2-s B) 1.3 x 10-7 1/M2-s C) 1.4 x 10-5 1/M2-s D) 1.3 × 10-4 1/M2-sarrow_forward3. Show the step-by-step process. Do not use shortcut methods. Make it as detailed as it can be. ENCODE THE ANSWER.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY